Abstract
Background
The two common sialic acid (Sia) in mammals are N-Acetylneuraminic acid (Neu5Ac) and its hydroxylated form N-Glycolylneuraminic acid (Neu5Gc). Unlike most mammals, humans cannot synthesize Neu5Gc that is considered foreign and recognized by circulating antibodies. Thus, Neu5Gc is a potential xenogenic carbohydrate antigen in bioprosthetic heart valves (BHV) that tend to deteriorate in time within human patients.
Methods
We investigated Neu5Gc expression in non-engineered animal-derived cardiac tissues and in clinically used commercial BHV, and evaluated Neu5Gc immunogenicity on BHV through recognition by human anti-Neu5Gc IgG.
Results
Neu5Gc was detected by immunohistochemistry in porcine aortic valves and in porcine and bovine pericardium. Qualitative analysis of Sia-linkages revealed Siaα2–3>Siaα2–6 on porcine/bovine pericardium while the opposite in porcine aortic/pulmonary valve cusps. Similarly, six commercial BHV containing either porcine aortic valve or porcine/bovine/equine pericardium revealed Siaα2–3>Siaα2–6 expression. Quantitative analysis of Sia by HPLC showed porcine/bovine pericardium express four-fold higher Neu5Gc levels compared to the porcine aortic/pulmonary valves, with Neu5Ac at six-fold over Neu5Gc. Likewise, Neu5Gc was expressed on commercial BHV (186.3±16.9 pmol Sia/μg protein), with Neu5Ac at eight-fold over Neu5Gc. Affinity-purified human anti-Neu5Gc IgG showing high specificity towards Neu5Gc-glycans (with no binding to Neu5Ac-glycans) on a glycan microarray, strongly bound to all tested commercial BHV, demonstrating Neu5Gc immune recognition in cardiac xenografts.
Conclusions
We conclusively demonstrated Neu5Gc expression in native cardiac tissues, as well as in six commercial BHV. These Neu5Gc xeno-antigens were recognized by human anti-Neu5Gc IgG, supporting their immunogenicity. Altogether, these findings suggest BHV-Neu5Gc/anti-Neu5Gc may play a role in valve deterioration warranting further investigation.
Keywords: Transplantation, valvular heart disease, immunology, xeno-antigens, Neu5Gc
Introduction
Heart valve diseases include several pathologies prevalent in ~2–3% of the population in developed countries [1,2]. Valvular stenosis is frequently diagnosed and result in valve narrowing that restricts normal blood flow with no available medicinal treatment but valve replacement [2]. Prosthesis selection includes either bioprosthetic or mechanical heart valves (BHV or MHV, respectively), however the latter requires supporting long-term anticoagulation. Some studies found better long-term survival for patients receiving the latest generation MHV over BHV [3], while others demonstrated equal benefit, but with higher incidence of stroke and bleeding in patients receiving MHV [4]. Use of BHV for younger patients is controversial [3-5], yet constantly increasing [3,6,7] especially with the relative success of the less invasive valve-in-valve trans catheter techniques [8,9] as a later procedure if BHV deteriorates. Given the tradeoff between the risk of reoperation because of BHV degeneration and the risk associated with long-term anticoagulation, most recent guidelines emphasize patients’ choice regarding the type of valve. However, MHV remains the recommended prosthesis in patients below the age of 60 if anticoagulation therapy can be properly maintained.
BHV made of animal-derived cardiac tissues (i.e. pericardium or porcine heart valve) are less durable compared to mechanical valves, largely due to structural valve deterioration (SVD) [10,11], especially in patients below 60 years of age [12,13]. In addition, valve deterioration is observed earlier in younger BHV recipients (under the age of 50), and high mortality is associated with reoperation due to SVD [5]. The immune response towards xeno-antigens has been suggested to participate in bioprosthesis SVD [14,15]. Galα1–3Gal (α-Gal) is widely accepted as an immunogenic glycan to which the human immune system is highly responsive [16,17], and this antigen was recently identified in BHV [18,19]. All humans have circulating anti-α-Gal antibodies that respond to this glyco-epitope on xenogenic tissues [16,20,21] causing in turn an antibody-mediated inflammation that may contribute to BHV degeneration [5,18]. However, studies using α-Gal-knockout tissues reduced immune response against BHV but did not eliminate it [5,22,23], prompting search for other non-Gal immunogens in BHV [5,24-26]. One such candidate non-Gal immunogen had been suggested to be the non-human sialic acid N-Glycolylneuraminic acid (Neu5Gc) carbohydrate xeno-antigen [1,23,26-30], that shares some similarities with α-Gal (neither are synthesized in humans due to inactivation of specific genes and the humoral response encompass IgA, IgG and IgM) [27].
Sialic acids (Sia) are 9-carbon backbone α-keto sugars covering the tips of sugar chains (glycans) on cell surface glycoproteins and glycolipids (glycoconjugates). The two most common Sias in mammals are N-Acetylneuraminic acid (Neu5Ac) and its hydroxylated form Neu5Gc. However, unlike most other mammals, humans cannot produce Neu5Gc due to a deletion in the CMAH gene encoding the CMP-Neu5Ac hydroxylase [31,32]. Therefore, Neu5Gc is foreign to humans, yet dietary consumption of mammalian food products (e.g. red meat and milk products) result in Neu5Gc intake and its expression on the surface of some human cells [32,33]. Various Neu5Gc-containing glycans (on human cells from dietary intake or intrinsically in xenografts) are recognized as foreign by the human immune system resulting in a diverse anti-Neu5Gc immune response [30,34] that can lead to chronic inflammation (e.g. in cancer and vascular disease) [35-37], and last for many years even after a short exposure to animal-derived tissues [38]. Neu5Gc had been detected in various porcine organs [28], and more recently described in porcine heart valves by immunohistochemistry [29] and mass spectrometry of glycoproteins [39], but not of glycolipids [24]. Therefore, Neu5Gc may be a non-Gal xeno-antigen in BHV potentially recognized by circulating human anti-Neu5Gc antibodies, thereby likely contributing to BHV structural deterioration. Here we aimed to qualitatively and quantitatively investigate Neu5Gc expression in various animal-derived cardiac tissues and in commercial BHV used in the clinic. We further tested the binding of highly specific human anti-Neu5Gc IgG antibodies to Neu5Gc-xeno-antigens present on all tested commercial BHV. Altogether these findings support a potential role for Neu5Gc and anti-Neu5Gc antibodies in BHV deterioration.
Materials and Methods
Human sera samples
Human sera were obtained from the Israeli Blood Bank and used in accordance with the Helsinki declaration and Tel Aviv University Institutional Review Board.
Tissue samples
Native porcine and bovine tissues were obtained from a local slaughterhouse. Pericardium and valve cusps (aortic and pulmonary) were carefully dissected and kept frozen at −20 °C separately until analysis, or fixed for immunohistochemistry as detailed. The commercial bioprostheses used in the study were kindly donated by the corresponding producing companies (Medtronic, Sorin, St. Jude Medical).
Antibodies and Lectins
We used affinity-purified polyclonal chicken anti-Neu5Gc IgY [40] (Biolegend), horse radish peroxidase (HRP)-streptavidin , Cy3-streptavidin, HRP-goat-anti-human IgG, Cy3-goat-anti-human IgG (H+L), biotinylated donkey-anti-chicken IgY, HRP-AffiniPure donkey-anti-chicken IgY (IgG)(H+L), Cy3-donkey-anti-chicken IgY (IgG)(H+L) (Jackson ImmunoResearch), biotinylated GSL I – isolectin B4 (IB4), biotinylated SNA (Sambucus nigra) and biotinylated MAL-II (Maackia amurensis lectin II) (Vector Labs). All antibodies and lectins were used at optimized saturating concentrations.
Immunohistochemistry
Porcine heart valve cusps and porcine and bovine pericardia (the full thickness parietal tissue) as well as porcine kidney (positive control) were fixed in buffered paraformaldehyde. Immunostaining was performed on 4 μm paraffin sections using cold water fish gelatin (Sigma) in PBS buffer containing 0.1% Triton X-100 as blocking reagent. Primary antibody was the affinity-purified polyclonal chicken anti-Neu5Gc IgY [40] (Biolegend) in serial dilutions where a dilution of 1:2000 was found to be optimal. Secondary antibody was biotinylated-donkey anti-chicken IgY (1:500; Jackson ImmunoReserach) detected by HRP-streptavidin (Jackson ImmunoResearch) followed by DAB reagent (Dako). Positive control tissue was porcine kidney and negative controls were obtained by omitting the primary antibody.
Tissue homogenization
Porcine, bovine or commercial BHV portions were prepared from frozen or refrigerated tissue samples, respectively. Tissues were thawed and weighed. Samples (~16–35 mg) were then finely sliced and dissolved in 1 ml TRIS-HCl buffer (50 mM pH5.5) with 10 mM of Ca2+. The solution was thoroughly vortexed for 30 seconds and then 2 mg/ml of collagenase type 2 (Sigma) was added and the mixture was incubated at 37 °C for 1 hour while shaking at 220 rpm. Samples were then put on ice and sonicated with a probe sonicator (Sonic dismembrator, Fisher scientific) three times at a medium power, each for 10 seconds with 30-second intervals incubation on ice. Sonicated solutions together with non-homogenized tissue were then inserted to a glass dounce tissue grinder (2 ml; Sigma) and homogenized with a loose pestle then with a tight pestle (10 times each). Of note, the fresh tissues were fully disrupted while BHV samples were more sturdy. The protein content in the homogenate was evaluated by a standard BCA assay according to manufactures’ protocol (Pierce). The homogenate was kept at −20 °C until use.
Sialic acid analysis by DMB-HPLC
Sia content of tissue and BHV homogenates was analyzed. Sias were released from glycoconjugates by acid hydrolysis; either by hydrolysis that removes O-Acetylation, with 0.1 M of H2SO4 for 1.5 hours (neutralizing with 0.1 M of NaOH) or by hydrolysis that maintains O-Acetylation with 2 M of acetic acid for three hours, both at 80 °C [41]. Free Sias were then derivatized with 1,2-diamino-4,5-methylenedioxybenzene (DMB; Sigma) for 2.5 hours at 50 °C, separated by Microcon-10 centrifugal filters and analyzed by fluorescence detection on reverse-phase high pressure liquid chromatography (DMB-HPLC) (Hitachi HPLC Chromaster). HPLC run was on C18 column (Phenomenex C18 Gemini 250 × 4.6 mm) at 24 °C in running buffer [84.5% ddH2O, 8.5% acetonitrile, 7% methanol (Merck)] for 60 minutes (min) at a flow rate of 0.9 ml/min. Quantification of Sias was done by comparison with known quantities of DMB-derivatized Neu5Ac [41].
ELISA
Binding of human sera, lectins or antibodies to tissue homogenates was tested by ELISA as described [34]. Tissue homogenates (entire crude suspension) were coated at optimized equal protein concentrations in duplicates at 2 μg/well in 50 mM sodium carbonate-bicarbonate buffer, pH 9.5 onto 96-well microtiter plates (Costar, Corning) and plates were incubated overnight at 4 °C. Wells were blocked for 1 hour at room temperature with blocking buffer [PBS pH 7.4, 1% ovalbumin (Grade V, Sigma)]. Wells were aspirated and incubated with diluted primary antibody 100 μl/well in the same blocking buffer for two hours at room temperature (1:100 diluted human or mouse serum samples, chicken anti-Neu5Gc IgY at 1:1000, biotinylated SNA or MAL-II at 1μg/ml). The plates were washed three times with PBST (PBS pH 7.4, 0.1% Tween) and subsequently incubated for 1 hour at room temperature with HRP-conjugated secondary antibody in PBS (respectively: HRP-goat anti-human IgG 0.11 μg/ml, goat-anti-mouse IgG 0.16 μg/ml, HRP-donkey-anti-chicken IgY 0.26 μg/ml and HRP-streptavidin 0.1 μg/ml). After washing three times with PBST, wells were developed with 140 μl of O-phenylenediamine in 100 mM citrate-PO4 buffer, pH 5.5, and the reaction stopped with 40 μl of H2SO4 (4 M). Absorbance was measured at 490 nm on SpectraMax M3 (Molecular Devices). Specific binding was defined by subtracting the background readings obtained with the secondary antibody only on coated wells.
Affinity purification of human anti-Neu5Gc IgG
Pooled human IgG, from clinical therapeutic IVIG leftovers (GAMMAGARD LIQUID, Baxter), was kindly provided by Dr. Adriana H. Tremoulet from the Rady Children's Hospital, San Diego, USA. Polyclonal human anti-Neu5Gc IgG were affinity-purified from IVIG on sequential columns with immobilized human serum and chimpanzee serum as previously described [34]. Chimpanzee sera were obtained from the local zoo only during routine maintenance procedures and kindly provided by Dr. Gillad Goldstein, curator of the Zoological Center Tel Aviv, Safari Park (Israel) and Dr. Nili Avni-Magen, Head Veterinarian and Zoological Director of The Tisch Family Zoological Gardens in Jerusalem (Israel).
Biotinylation of affinity-purified human anti-Neu5Gc IgG
Antibodies were biotinylated according to the manufacturer's instruction (EZ-Link™ Sulfo-NHS Biotin kit, Pierce). Briefly, 0.5 ml of purified anti-Neu5Gc IgG 0.5 mg/ml were supplemented with 3 μl 10 mM of sulfo-NHS-biotin and incubated on ice for two hours. Non-reacted Sulfo-NHS-biotin was removed using zeba-spin gel filtration columns (Pierce).
Sialoglycan microarray fabrication
Arrays were printed on epoxide-derivatized Corning slides as described (Array1 [42]) with some modifications. Arrays were fabricated with NanoPrint LM-60 Microarray Printer (Arrayit) on epoxide-derivatized slides (Corning) with 16 sub-array blocks on each slide. Glycoconjugates were distributed into one 384-well source plates using 4 replicate wells per sample and 8 μl per well (Version 1.0). Each glycoconjugate was prepared at 100 μM in an optimized print buffer (300 mM phosphate buffer, pH 8.4). To monitor printing quality, replicate-wells of human IgG (Jackson, at 200, 100, 50, 25, 12.5, 6.25 ng/μl in PBS+10% glycerol) and AlexaFlour-555-Hydraside (Invitrogen, at 1 ng/μl in 178 mM phosphate buffer, pH 5.5) were used for each printing run. The arrays were printed with four 946MP3 pins (5 μm tip, 0.25 μl sample channel, ~100 μm spot diameter; Arrayit). Each block (sub-array) has 17 spots/row, 20 columns with spot to spot spacing of 225 μm. The humidity level in the arraying chamber was maintained at about 66% during printing. Printed slides were left on arrayer deck over-night, allowing humidity to drop to ambient levels (40-45%). Next, slides were packed, vacuum-sealed and stored in a desiccant chamber at room temperature (RT) until used.
BHV microarray fabrication
Arrays were printed with NanoPrint LM-60 Microarray Printer (Arrayit) on epoxide-derivatized slides (Corning) with 16 sub-array blocks on each slide. Homogenates of BHV were distributed into one 384-well source plates using 4 replicate wells per sample and 8 μl per well. Each BHV's homogenate was centrifuged and the supernatant diluted in PBS pH 7.4 to 100 ng/μl. To monitor printing quality, human IgG (Jackson, 40 ng/μl in PBS+10% glycerol) and AlexaFlour-555-Hydraside (Invitrogen, at 1 ng/μl in 178 mM phosphate buffer, pH 5.5) were used for each printing run. The arrays were printed with one 946MP3 pin (5 μm tip, 0.25 μl sample channel, ~100 μm spot diameter; Arrayit). Each block (sub-array) has 14 rows, 6 columns with spot to spot spacing of 225 μm. The humidity level in the arraying chamber was maintained at about 70% during printing. Printed slides were left on arrayer deck over-night, allowing humidity to drop to ambient levels (40–45%). Next, slides were packed, vacuum-sealed and stored in a desiccant chamber at RT until used.
Sialoglycan microarray binding assay
Slides were developed and analyzed as previously described [42]. Slides were rehydrated with dH2O and incubated for 30 min in a staining dish with 50°C pre-warmed ethanolamine (0.05 M) in Tris-HCl (0.1 M, pH 9.0) to block the remaining reactive epoxy groups on the slide surface, then washed with 50 °C pre-warmed dH2O. Slides were centrifuged at 200×g for three min then fitted with ProPlate™ Multi-Array 16-well slide module (Invitrogen) to divide into the sub-arrays (blocks). Slides were washed with PBST, aspirated and blocked with 200 μl/sub-array of blocking buffer (PBS/OVA, 1% w/v ovalbumin, Sigma in PBS, pH 7.3) for 1 hour at RT with gentle shaking. Next, the blocking solution was aspirated and 200 μl/ block of primary detection diluted in PBS/OVA was added: polyclonal affinity-purified human anti-Neu5Gc IgG (from IVIG) at 40 μg/ml, biotinylatedlectins (SNA/MAL-II/IB4; 20 μg/ml). Primary detections were incubated with gentle shaking for 2 hours at RT. Slides were washed three times with PBST (PBS, 0.1% Tween) then with PBS for 10 min/wash with shaking. Bound antibodies were detected by incubating with secondary detection diluted in PBS, 200 μl/block at RT for 1 hour: Cy3-goat-anti-human IgG (H+L) (0.4 μg/ml) or Cy3-streptavidin (1.2 μg/ml). Slides were washed three times with PBST then with PBS 10 min/wash followed by removal from ProPlate™ Multi-Array slide module and immediately dipping slide in a staining dish with dH2O for 10 min with shaking, then centrifuged at 200×g for 3 min. Dry slides were vacuum-sealed and stored in dark until scanning.
Array slide processing
Processed slides were scanned and analyzed as described at 10 μm resolution with a Genepix 4000B microarray scanner (Molecular Devices) using 350 gain [42]. Image analysis was carried out with Genepix Pro 6.0 analysis software (Molecular Devices). Spots were defined as circular features with a variable radius as determined by the Genepix scanning software. Local background subtraction was performed.
Statistical analysis
Statistical analyses were performed using GraphPad Prism 5.0, and described in context in the figure legends.
Results
Expression of Neu5Gc in native untreated porcine and bovine cardiac tissues
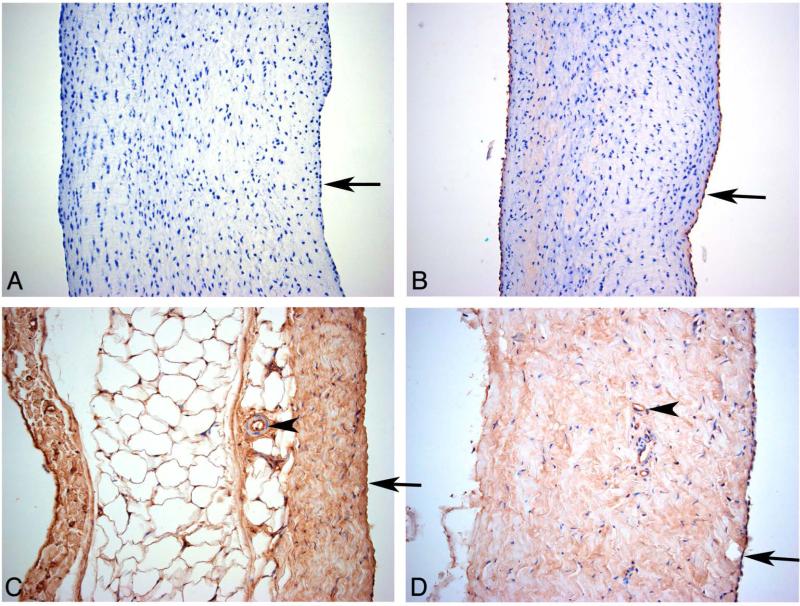
To evaluate Neu5Gc expression in porcine aortic valvular cusp and porcine and bovine pericardium, the main source of biological tissues for commercial BHV, immunohistochemistry analysis was performed using a highly specific polyclonal antibody that recognize multiple Neu5Gc-containing glycans [40,42]. Anti-Neu5Gc antibody showed a clear staining of the porcine valve cusp endothelium (Figure 1B) and a very faint staining in the cusp matrix tissue under the endothelium. Porcine pericardium (Figure 1C) showed staining of the entire matrix tissue with an increased staining of the mesothelial cells. The endothelium of a small artery (Figure 1C) is positive while the arterial muscular cells are negative. Bovine pericardium (Figure 1D) shows similar staining to the porcine pericardium with diffused staining of the matrix and strong staining of the epicardial and vascular endothelial cells. The negative controls, omitting the primary antibody, were always completely negative (Figure 1A). Porcine kidney positive control tissue (not shown) showed strong anti-Neu5Gc staining of all vascular endothelial cells (artery, glomerular- and peritubular-capillaries). The tubular cell brush border was positive and a weak granular staining of the tubular cell cytoplasm was also seen. All artery muscular cells were completely negative, as seen for the pericardial small arteries in Figures 1C–D, showing that the diffuse staining of the pericardium matrix is not due to non-specific background staining. Altogether, Neu5Gc seems to be intrinsically expressed on all tested animal-derived cardiac tissues that make up various commercial BHV.
Figure 1.
Immunohistochemical investigation of porcine and bovine tissues using anti-Neu5Gc antibody. (A) Porcine aortic valve cusp negative control. Porcine aortic valve cusp (B) and pericardium (C) and bovine pericardium (D) were stained for Neu5Gc. Arrows show the endothelium (A, B) and epicardium (C, D) and arrowheads show a small artery (C) and a capillary (D) with a strong staining of their endothelium while the muscular cells are completely negative. Fragments of tissues were used for analysis; data is representative of at least two independent experiments.
Characterization of Neu5Gc antigen in native untreated porcine and bovine cardiac tissues
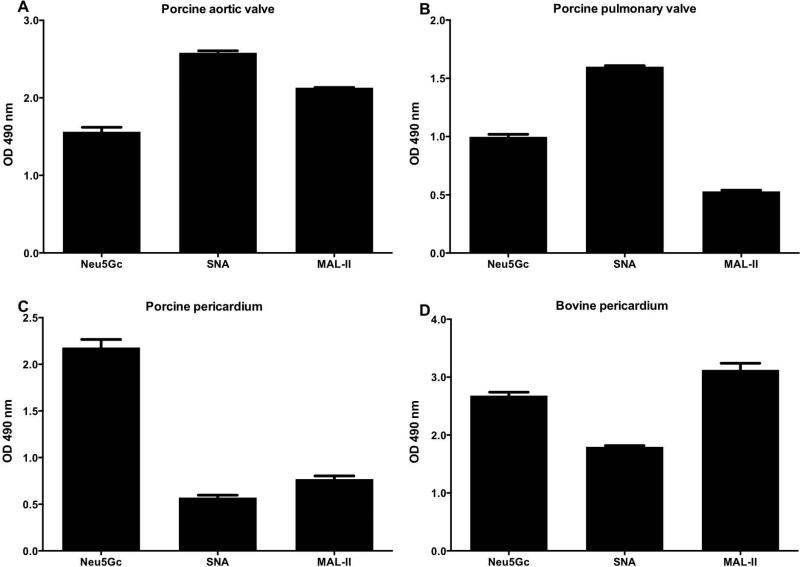
Neu5Ac and Neu5Gc are Sia normally covering the tips of glycans on various glycoproteins and glycolipids on the cell surface [32]. Sias are attached to underlying carbohydrate core chains via α2–3 or α2–6 linkages (namely, Sia carbon C-2 attached to C-3 or C-6 of galactose or N-Acetylgalactosamine). To further characterize Sia expression and their core saccharide linkage in xenogenic cardiac tissues of relevance for BHV, we analyzed porcine aortic and pulmonary valve cusps, and porcine and bovine pericardium (Figure 2). The tissues were partially homogenized and Sia distribution investigated by ELISA using anti-Neu5Gc IgY and Sia-binding lectins that can differentially recognize glycans carrying Siaα2–3 (MAL-II) or Siaα2–6 (SNA) [42]. This revealed that all tissues express Neu5Gc (Figure 2), supporting the immunohistochemistry analysis. Furthermore, both Siaα2–3 and Siaα2–6 linkages were found in all tissues analyzed (Figure 2) and showed a slightly different distribution. While porcine aortic or pulmonary valve cusps expressed higher levels of Siaα2–6 (SNA reactivity) compared to Siaα2–3 (MAL-II reactivity) (Figure 2A–B), the porcine and bovine pericardia showed a reversed expression pattern with Siaα2–3 expression more pronounced (Figure 2C–D). Overall, this immunological analysis revealed qualitative differences in Sia expression between the various cardiac tissues, with all showing Neu5Gc expression.
Figure 2.
Characterization of sialic acids in xenogenic cardiac tissues by ELISA. Tissue homogenates of native porcine aortic valve cusp (A), pulmonary valve cusp (B), pericardium (C), and bovine pericardium (D) were coated onto a 96-well plate and analyzed with anti-Neu5Gc IgY (Neu5Gc), biotinylated-SNA lectin or biotinylated-MAL-II lectin (detect α2–6-linked or α2–3-linked sialic-acids, respectively), washed then detected with HRP-anti-chicken IgY or HRP-streptavidin, respectively. All tissue samples express Neu5Gc, with higher levels in pericardium compared to valve cusps (at least two different samples from each tissue were tested, data represent three independent experiments; mean ± SEM).
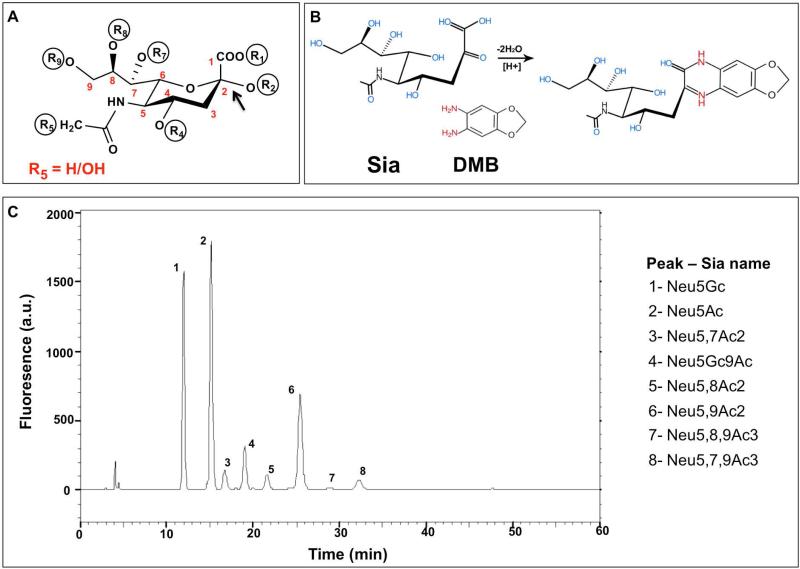
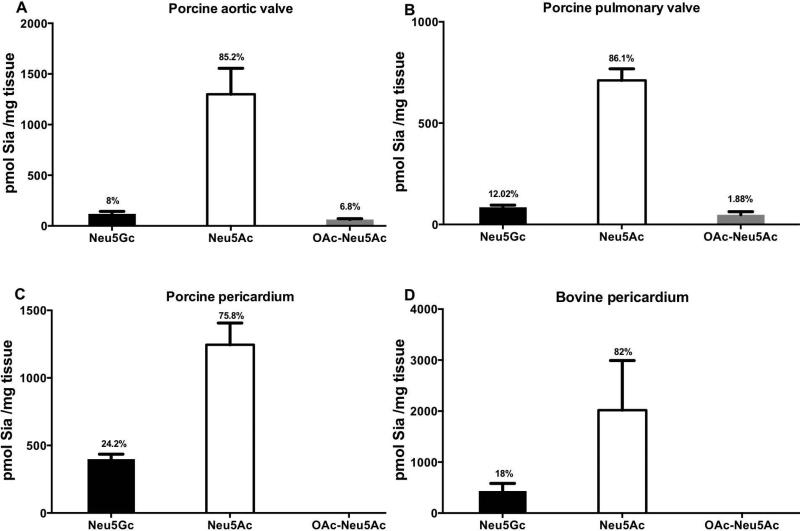
Sias can be further diversified with O-acetyl groups at positions C-4, C-7, C-8 or C-9 (Figure 3A) potentially adding to immunogenic Sia determinants. To directly identify and quantitate the various Sias (Neu5Ac, Neu5Gc and their O-Acetylation derivatives) in the valve cusps and pericardium, we further analyzed the homogenates by a sensitive fluorometric HPLC method (DMB-HPLC). In this analysis, Sia was released from glycoconjugates by mild acid hydrolysis followed by labeling with the fluorogenic reagent 1,2-diamino-4,5-methylenedioxybenzene (DMB) that reacted with α-keto acids (Figure 3B). DMB-labeled Sias were then separated on reverse-phase HPLC according to their hydrophobicity (e.g. Neu5Ac is more hydrophobic compared to its hydroxylated derivative Neu5Gc, thus migrating slower on the column; Figure 3C) using Sia-derivatives released from bovine submaxillary mucin (BSM) as reference for Sia type characterization (Figure 3B) [41]. Direct quantification of Sia by DMB-HPLC analysis revealed Neu5Gc expression in all xenogenic cardiac tissues assessed (Figure 4), confirming the previous immunological analysis (Figures 1 and 2). Furthermore, both porcine pericardium and bovine pericardium expressed four-fold higher Neu5Gc levels (416.3±17.8 pmol Sia/mg tissue; n=2, mean ± SEM of values in each of the pericardia; Figure 4C-D) compared to the porcine aortic/pulmonary valve cusps (101.8±17 pmol Sia/mg tissue; n=2, mean ± SEM of values in each of the valve cusps; Figure 4A-B). In addition, a considerably increased Neu5Ac expression (82.3±2.3 %; n=4, mean ± SEM of values in each of the tissues) compared to Neu5Gc (15.6±3.5 %; n=4, mean ± SEM of values in each of the tissues) were found in all tissues corresponding to a roughly six-fold difference (6.4±1.7; n=4, mean ± SEM of values in each of the tissues); O-Acetylated Neu5Gc was lacking in all tissues, while very low O-Acetylated Neu5Ac (4.3±3.5 %; n=2, mean ± SEM of values in each of the tissues) was found only in the porcine valves but not in porcine and bovine pericardia (Figure 4A–D). Altogether, our data demonstrate that Neu5Gc is an integral part of porcine/bovine cardiac tissues, especially in pericardium, that serve as the source for commercial BHV implanted in patients.
Figure 3.
Sialic acids analysis by DMB-HPLC of Bovine Submaxilary Mucins (BSM). (A) Schematic representation of Sia structure, the backbone carbons are numbered C-1 through C-9. The carboxylate group R1 is negatively charged at neutral pH; Sia is linked to underlying glycans through R2; R5 marks the difference between Neu5Gc (R5=OH) and Neu5Ac (R5=H). Positions R4, R7, R8, R9 can either carry hydroxyl or be modified with O-acetyl group. (B) Sia derivatized with 1,2-diamino-4,5-methylenedioxybenzene (DMB) becomes fluorescent. (C) Sialic acids profile of Bovine Submaxilary Mucins. BSM was hydrolyzed by mild acid hydrolysis (2 M acetic acid that preserves O-Acetylation) then DMB-derivatized and analyzed by reverse-phase HPLC. The analysis shows unique fluorescence peaks for each type of Sia at different retention times, based on hydrophobicity.
Figure 4.
Quantitative analysis of sialic acids in xenogenic cardiac tissues by DMB-HPLC. Neu5Ac, Neu5Gc and their O-Acetylated derivatives were quantified by DMB-HPLC in native porcine aortic valve cusp (A), pulmonary valve cusp (B), and pericardium (C), and bovine pericardium (D). 2 μl of tissue homogenates were acid hydrolyzed to release Sia followed by DMB-labeling and HPLC analysis. Sia content was quantified according to a standard curve of purified Neu5Ac. Percentage of each Sia from the total detected is indicated on the charts. All detected O-Acetylation types were summarized (at least two different samples from each tissue were tested, data represent at least two independent experiments; mean ± SEM).
Characterization of Neu5Gc antigen in clinically used commercial BHV
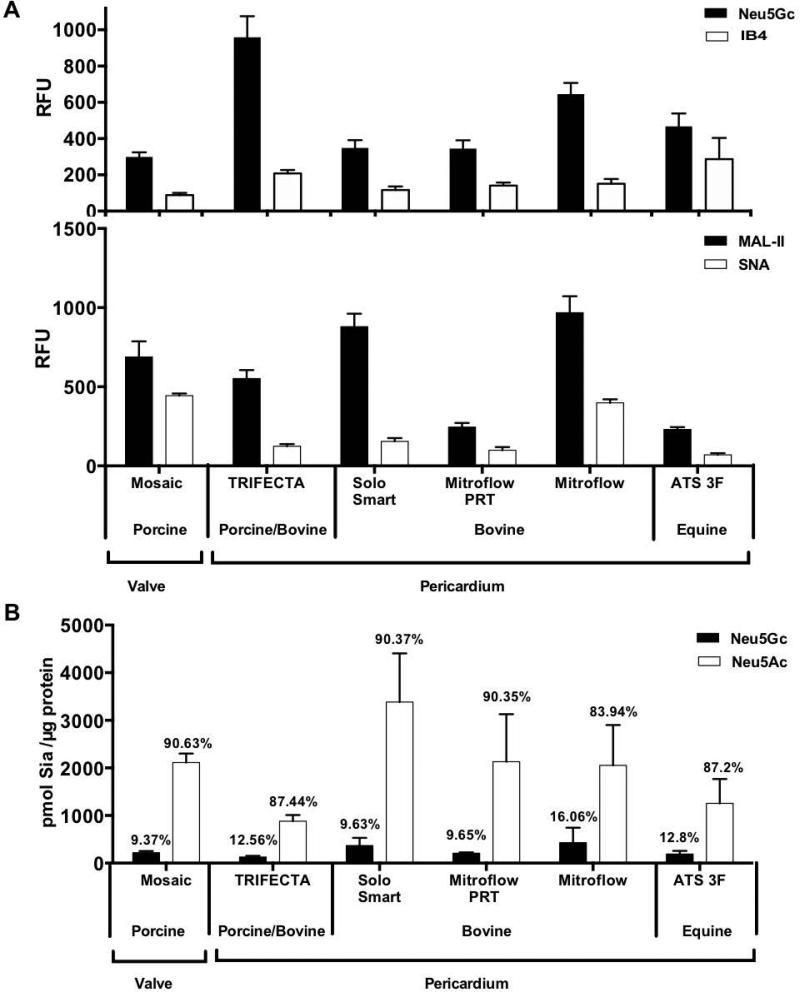
During manufacturing of BHV for clinical use, the animal-derived cardiac tissues are often processed to reduce immunogenicity and extend preservation (Table I). This may include pretreatment with: glutaraldehyde, formaldehyde and ethanol, anti-calcification and phospholipid-reduction treatments [1,43]. To determine Neu5Gc expression in various commercial BHV, six commercial commonly used BHV made up of porcine aortic valve (Mosaic) or porcine, bovine or equine pericardium were analyzed (Table I). Initial qualitative analysis of BHV homogenates showed all six samples express Neu5Gc, α-Gal (IB4) and both Siaα2–3 and Siaα2–6 linkages (Figure 5A). Interestingly, Neu5Gc RFU seemed 3 fold higher compared to α-Gal (3.3±0.5; n=6, mean ratio (Neu5Gc/IB4) ± SEM), however the values are not normalized according to a common standard curve. Those made of pericardium displayed four fold higher levels of Siaα2–3 (MAL-II) compared to Siaα2–6 (SNA) (3.7±0.6; n=5, mean ratio (Siaα2–3/Siaα2–6) ± SEM). This was consistent with the analysis of porcine and bovine pericardium (Figure 4C-D). Similarly, the Mosaic BHV made of porcine heart valve had higher levels of Siaα2–3, albeit to a lower extent (1.6 fold Siaα2–3 over Siaα2–6; Figure 5A). Interestingly, BHV from the same source showed different levels of lectin binding, probably reflecting differential BHV pretreatment during manufacturing (Figure 5A), or alternatively could be a consequence of different sources of cattle used. Hence, Mitroflow PRT that showed reduced Siaα2–3/6 compared to the original Mitroflow BHV, is known to be pre-treated with octanediol (amphipatic long chain alcohol with a hydrophobic tail and a hydrophilic head) to remove phospholipids that likely also affects sialo-glycoproteins or sialo-glycospingolipids content. BHV were more difficult to process compared with the fresh tissues, yet we used optimized equal amounts of protein in these qualitative measurements. This may result in underestimation of Sia in BHV compared with the fresh tissues using these assays. To directly characterize and quantify Neu5Gc and Neu5Ac expression in the BHV, DMB-HPLC analysis was performed. In this assay complete release of the total Sia is achieved and therefore provide a more accurate quantitative comparison between the different samples. This showed that regardless of the tissue source or the BHV processing procedure (Table I), Neu5Gc remained expressed (193.8±167.8 pmol Sia/μg protein; n=6, mean±SEM; Figure 5B), albeit at two-fold lower levels compared to the native xenogenic cardiac tissues (Figure 4C–D). Similar to the porcine and bovine native tissues, commercial BHV expressed higher levels of Neu5Ac (88.3±1.1 %) compared to Neu5Gc (11.6±1.1 %), corresponding to an eight-fold difference (8.0±0.7; Figure 5B). Thus, Neu5Gc is clearly expressed in the tested commercial BHV, despite pre-processing (Table I) for reduced immunogenicity. Yet it is possible that different batches of the same BHV brand would express variable levels of Sia.
Table I.
List of bioprosthetic heart valves (BHV) used in this study.
| Manufacturer | Name of BHV | Animal source | Bioprosthesis treatment | |
|---|---|---|---|---|
| 1 | Medtronic | Mosaic | Porcine aortic valves | Glutaraldehyde and alpha amino oleic acid |
| 2 | St Jude Medical | TRIFECTA | *Bovine and porcine pericardium | Linx™ AC Technology (proprietary anti-calcification treatment) |
| 3 | Sorin | Solo Smart | Bovine pericardium | Homocysteic acid |
| 4 | Sorin | Mitrofow PRT | Bovine pericardium | Octanediol |
| 5 | Sorin | Mitrofow | Bovine pericardium | Glutaraldehyde |
| 6 | Medtronic | ATS 3f | Equine pericardium | Glutaraldehyde |
The TRIFECTA valve (St. Jude Medical) is a three-leaflet stented bovine pericardial valve designed for supra-annular placement in the aortic position. In TRIFECTA, the valve leaflets are made of bovine pericardium. The stent, excluding the sewing cuff, is covered with porcine pericardium
Figure 5.
Analysis of Sia expression in commercial BHV by microarray and by DMB-HPLC. (A) Indicated BHV homogenates (100 ng/μl) were printed at ten replicates on epoxide-coated slides and detected for Neu5Gc, α-Gal, α2–3-linked or α2–6-linked sialic acids by Cy3-anti-Neu5Gc IgY (Neu5Gc) or biotinylated-IB4/MAL-II/SNA lectins, respectively, and binding detected by Cy3-strepavidin. Intensity unit are Relative Fluorescence Units (RFU; representing fluorescence at 532 nm after local background subtraction (representative of two independent experiments; mean ± SD). All BHV showed more Neu5Gc than α-Gal and more Siaα2–3 than Siaα2–6. (B) BHV homogenates were acid hydrolyzed with sulfuric acid and analyzed by DMB-HPLC for quantifying Neu5Ac and Neu5Gc (single BHV samples of each brand; two independent experiments; mean ± SEM). Neu5Gc is clearly detected in all tested BHV.
Human anti-Neu5Gc IgG reactivity against commercial BHV
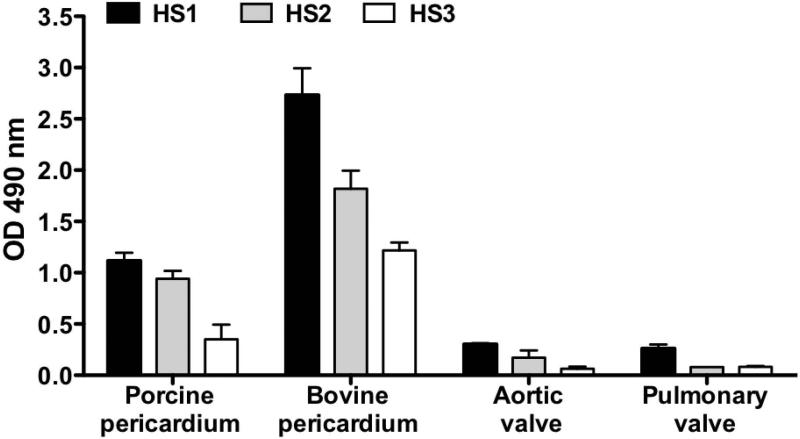
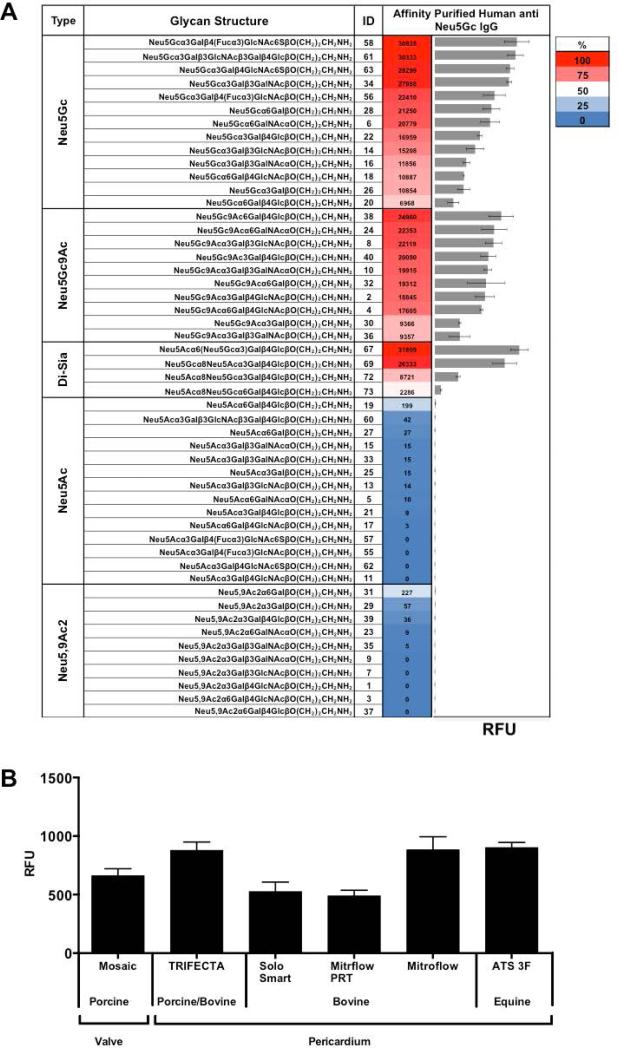
Humans express variable levels of serum anti-Neu5Gc antibodies (IgG, IgA, and IgM) recognizing multiple Neu5Gc-containing epitopes [34]. Sera from three individual healthy donors with anti-Neu5Gc IgG reactivity (data not shown) showed binding to porcine and bovine cardiac tissues (Figure 6). Human serum immunoglobulins could potentially recognize immunogenic epitopes also on commercial BHV, and some of it may be related to Neu5Gc/anti-Neu5Gc recognition. Therefore, polyclonal anti-Neu5Gc IgG was affinity-purified from clinical remnants of IVIG that is human IgG pooled from thousands of healthy donors, thus reflecting the average population reactivity. The specificity of the purified antibodies was determined on a glycan microarray representing the various cell surface sialoglycans, presenting multiple Siaα2–3/6 glycans with terminal Neu5Ac or Neu5Gc and their O-Acetylated derivatives (Figure 7A). The antibodies show a very strong specificity to Neu5Gc-glycans and, as expected, no cross-reactivity with the matching Neu5Ac-glycans, despite the fact that the only difference between matched pairs of Neu5Ac/Neu5Gc-glycans is a single oxygen atom on C-5 (Figure 3A). In addition, the polyclonal antibodies recognize Neu5Gcα2–3/6-linked, with or without C-9 O-Acetylation (Figure 7A). Subsequently, we tested the binding of these highly specific human anti-Neu5Gc IgG antibodies to the commercial BHV homogenates, revealing they could all be recognized (Figure 7B). Of note, the Mitroflow PRT showed reduced anti-Neu5Gc IgG binding compared to other BHV. Yet despite the much higher Neu5Ac expression and the overall reduction in Neu5Gc content on the commercial BHV compared to the native xenogenic cardiac tissues, it seemed that the manufacturing process did not eliminate the antibodies binding to Neu5Gc on commercial BHV.
Figure 6.
Human sera reactivity with porcine and bovine cardiac tissues. Tissue samples from native porcine aortic valve, pulmonary valve, and pericardium, and bovine pericardium were coated on 96-well microplates (1 μg/well) and serum of three individual healthy human donors (HS1, HS2, HS3) were tested at 1:100 dilution (two independent experiments; mean ± SEM).
Figure 7.
Binding of affinity-purified polyclonal human anti-Neu5Gc IgG on sialic acid glycan-microarray and BHV microarray. Human anti-Neu5Gc IgG were affinity-purified from clinically used IVIG (pooled human IgG). 40 ng/μl were tested on glycan microarray (A) and BHV microarray (B) followed by detection with Cy3-anti-human IgG (Intensity unit are Relative Fluorescence Units (RFU; representing fluorescence at 532 nm after local background subtraction; representative of two independent experiments; mean ± SD)
Discussion
Here we qualitatively and quantitatively demonstrate Neu5Gc expression in various animal-derived cardiac tissues and in six commercial BHV used in the clinic. We further show that highly specific human anti-Neu5Gc IgG antibodies recognize Neu5Gc-xeno-antigens present on six commercial BHV tested. Altogether these findings support a role of Neu5Gc and anti-Neu5Gc antibodies in BHV immunogenicity, which may be involved in progressive tissue failure. Although SVD occurs early in some bioprostheses, it is generally considered to begin 7 to 8 years after implantation and its occurrence increases rapidly after 10 years [44]. Age is a clear factor of SVD that ensues earlier in young patients. Progressive valve stenosis related to fibrosis and leaflets calcification are the main causes of SVD, while leaflet tear with acute or subacute valvular regurgitation is less frequent [44]. According to current knowledge, SVD mechanism can be due to 3 different biological processes: First, glutaraldehyde treatment to reduce antigenicity of xenogeneic bioprosthetic tissue may predispose it to a passive degenerative process with calcium crystal formation, and accumulation is partially prevented by anti-calcification treatment; Second, the atherosclerotic process involved at least in part in native aortic valve degeneration, is also likely playing a role in bioprosthesis SVD. In support, recent studies demonstrated a relation between classical cardiovascular risk factors such as diabetes, metabolic syndrome, dyslipidemia, or smoking and SVD; Third, and as suggested in the present study, bioprosthetic valve tissue does remain immunogenic, and this immunogenicity might elicit, or at least participate in, an inflammatory process that promotes progressive tissue degeneration [44].
Species-specific molecules in xenografts are recognized as immunogenic non-self and elicit an immune response to eliminate these targets [45]. Clinically used BHV are made of animal-derived cardiac tissues that are processed to reduce immunogenicity, but are nevertheless relatively short-lived [1]. In addition to protein xeno-antigens, carbohydrate antigens also play a major role and include the α-Gal [16] or non-Gal immunogens [5,24,25,46,47]. Neu5Gc had been suggested to be a major non-Gal xeno-antigen in BHV [1,27-29,46], thus prompting further in-depth investigation.
Here, for the first time, we conclusively demonstrated and quantitated Neu5Gc expression in porcine and bovine cardiac tissues as well as in six commercial BHV from 3 different species, namely porcine, bovine and equine (of note, we tested one sample of each BHV). Analysis of native pericardium and BHV made of pericardium showed specific Sia-linkage preferences towards Siaα2–3>Siaα2–6, which may become important for future therapeutic approaches (e.g. for designing potent inhibitors for specific immune reactants). In all cardiac tissues Neu5Gc was markedly lower than Neu5Ac and yet preserved its immunogenicity allowing recognition by polyclonal human anti-Neu5Gc IgG representing the average population response. Thus, Neu5Gc-glycoconjugates are likely candidates to become a major factor contributing to BHV deterioration in patients. Quantification of xeno-antigens in BHV is important for quality control, risk evaluation and patients’ confidence in BHV choice. While BHV had been used in the clinic for many years, the major α-Gal xeno-antigen had only recently been quantitated [19]. Quantification of Neu5Gc in commercial BHV performed in this work is of clear importance and provides initial lead to manage its immunogenicity, as would be for other potential antigenic structures. Neu5Gc and α-Gal are both carbohydrate xeno-antigens. However, while α-Gal is represented by the single glycan Galα1–3Gal, Neu5Gc-containing antigens encompass a large collection of xeno-antigens epitopes with terminal Neu5Gc [27,32,34], some represented on the sialoglycan microarray (Figure 7A). Adding to the complexity, both xeno-antigens can be conjugated to proteins or lipids that may further affect their immunogenicity [27]. Interestingly, Neu5Gc-containing glycosphingolipids were not detected in porcine heart valve cusps [24] suggesting Neu5Gc-antigens are mainly on glycoproteins. It would be interesting to further investigate Neu5Gc content specifically in collagen that is a major glycoprotein constituent in BHV tissue. In addition, investigating batch-to-batch variability in xenoantigens expression on commercial BHV is warranted.
All humans have pre-existing circulating IgA/IgG/IgM antibodies against glycoconjugates with terminal α-Gal or Neu5Gc. Yet, anti-Neu5Gc antibodies are quite diverse in their levels and binding patterns between individuals, some circulate at very high levels [34]. The co-localization of each xeno-antigen and its corresponding anti-Neu5Gc antibodies within a given patient likely mediates the potential deleterious effects of immune response against the xenografts by chronic inflammation or by various routes (e.g. immune complex formation, atherosclerosis-like, etc) [28,45]. α-Gal/anti-Gal antibodies had been shown to mediate inflammation leading to increased BHV calcification [21,48,49], and implanted BHV further induced anti-Gal response [18,21]. It had already been shown that co-existing Neu5Gc/anti-Neu5Gc antibodies mediate chronic inflammation in cancer [36,37] and atherosclerosis [35]. Likewise, it is possible that chronic inflammation mediated by Neu5Gc/anti-Neu5Gc antibodies would potentially be involved in BHV deterioration in patients. Thus both anti-Gal antibodies and anti-Neu5Gc antibodies may contribute to valve deterioration. Of note, either pre-existing anti-Neu5Gc antibodies or those possibly induced after exposure to xenograft may contribute to the process of valve deterioration. Therefore, evaluation of anti-Neu5Gc antibodies in patients before and after BHV surgery could possibly allow to better evaluate associated risks. The difficulties in obtaining allografts naturally prompted search of improved xenograft, in which most antigens had been removed either chemically or by generating unique knockout strains that lack xeno-antigen expression, including the α-Gal-deficient [22,49] and the double-knockout α-Gal/Neu5Gc-deficient porcine strains [50], that are currently being investigated [1]. It would be interesting to test the presence of these antigens in those strains especially in valve and pericardium. Future studies are required to evaluate the role of Neu5Gc immunogenicity in human patients and its potential role in bioprosthesis SVD.
Acknowledgments
The authors are indebted to Johan Mölne, department of Pathology, Sahlgrenska University Hospital for immunohistochemical evaluation. We thank Laia Muixí for valuable technical assistance. V.P-K supervised the research and designed the studies; E.M.R., S.L.B-A and M.E.B. performed the research; T.M., H.Y., I.F., C.C, M.G, J-C.R. and X.C. provided crucial reagents; E.M.R., S.L.B-A and V.P-K. wrote the paper; and all authors read and approved the manuscript.
Grant support
This work was supported in part by a grant from the Israeli National Nanotechnology Initiative and Helmsley Charitable Trust for a Focal Technology Area on Nanomedicines for Personalized Theranostics (to V.P-K); The European Commission's 7th Framework Programme Marie-Curie grant PIIF-GA-2012-327726 (to V.P-K); and the European Commission's 7th Framework Programme FP7-Health-2013-INNOVATION-1, “Translink Defining The Role Of Xeno-Directed And Autoimmune Events In Patients Receiving Animal-Derived Bioprosthetic Heart Valves”, Grant agreement no: 603049 (V.P-K, M.E.B., C.C., M.G., R.M., J-C.R., T.L.T., X.C., JP.S., E.C.); National Institutes of Health grant R01GM076360 (to X.C.); and Governmental grants to the Sahlgrenska University Hospital.
Abbreviations
- Sia
Sialic acid
- Neu5Ac
N-Acetylneuraminic acid
- Neu5Gc
N-Glycolylneuraminic acid
- BHV
bioprosthetic heart valves
- MHV
mechanical heart valves
- SVD
structural valve deterioration
- α-Gal
Galactoseα1–3Galalactose; Galα1–3Gal
- HRP
horse radish peroxidase
- DMB
1,2-diamino-4,5-methylenedioxybenzene
References
- 1.MANJI RA, LEE W, COOPER DK. Xenograft bioprosthetic heart valves: Past, present and future. Int J Surg. 2015;23:280–284. doi: 10.1016/j.ijsu.2015.07.009. [DOI] [PubMed] [Google Scholar]
- 2.D'ARCY JL, PRENDERGAST BD, CHAMBERS JB, RAY SG, BRIDGEWATER B. Valvular heart disease: the next cardiac epidemic. Heart. 2011;97:91–93. doi: 10.1136/hrt.2010.205096. [DOI] [PubMed] [Google Scholar]
- 3.SURI RM, SCHAFF HV. Selection of aortic valve prostheses: contemporary reappraisal of mechanical versus biologic valve substitutes. Circulation. 2013;128:1372–1380. doi: 10.1161/CIRCULATIONAHA.113.001681. [DOI] [PubMed] [Google Scholar]
- 4.CHIKWE J, CHIANG YP, EGOROVA NN, ITAGAKI S, ADAMS DH. Survival and outcomes following bioprosthetic vs mechanical mitral valve replacement in patients aged 50 to 69 years. JAMA. 2015;313:1435–1442. doi: 10.1001/jama.2015.3164. [DOI] [PubMed] [Google Scholar]
- 5.MANJI RA, EKSER B, MENKIS AH, COOPER DK. Bioprosthetic heart valves of the future. Xenotransplantation. 2014;21:1–10. doi: 10.1111/xen.12080. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.BROWN JM, O'BRIEN SM, WU C, et al. Isolated aortic valve replacement in North America comprising 108,687 patients in 10 years: changes in risks, valve types, and outcomes in the Society of Thoracic Surgeons National Database. J Thorac Cardiovasc Surg. 2009;137:82–90. doi: 10.1016/j.jtcvs.2008.08.015. [DOI] [PubMed] [Google Scholar]
- 7.DUNNING J, GAO H, CHAMBERS J, et al. Aortic valve surgery: marked increases in volume and significant decreases in mechanical valve use--an analysis of 41,227 patients over 5 years from the Society for Cardiothoracic Surgery in Great Britain and Ireland National database. J Thorac Cardiovasc Surg. 2011;142:776–782.e3. doi: 10.1016/j.jtcvs.2011.04.048. [DOI] [PubMed] [Google Scholar]
- 8.WEBB JG, DVIR D. Is Transcatheter Aortic Valve Replacement a Durable Therapeutic Strategy. JACC Cardiovasc Interv. 2015;8:1092–1094. doi: 10.1016/j.jcin.2015.04.013. [DOI] [PubMed] [Google Scholar]
- 9.ROZEN G, FEFER P, SHINFELD A, et al. The changing characteristics and outcomes of patients undergoing surgical aortic valve replacement in the transcatheter aortic valve implantation era. J Cardiovasc Med (Hagerstown) 2015;16:261–266. doi: 10.2459/JCM.0000000000000097. [DOI] [PubMed] [Google Scholar]
- 10.RAHIMTOOLA SH. Choice of prosthetic heart valve in adults an update. J Am Coll Cardiol. 2010;55:2413–2426. doi: 10.1016/j.jacc.2009.10.085. [DOI] [PubMed] [Google Scholar]
- 11.SIDDIQUI RF, ABRAHAM JR, BUTANY J. Bioprosthetic heart valves: modes of failure. Histopathology. 2009;55:135–144. doi: 10.1111/j.1365-2559.2008.03190.x. [DOI] [PubMed] [Google Scholar]
- 12.DAVID TE, FEINDEL CM, BOS J, IVANOV J, ARMSTRONG S. Aortic valve replacement with Toronto SPV bioprosthesis: optimal patient survival but suboptimal valve durability. J Thorac Cardiovasc Surg. 2008;135:19–24. doi: 10.1016/j.jtcvs.2007.04.068. [DOI] [PubMed] [Google Scholar]
- 13.JOHNSTON DR, SOLTESZ EG, VAKIL N, et al. Long-term durability of bioprosthetic aortic valves: implications from 12,569 implants. Ann Thorac Surg. 2015;99:1239–1247. doi: 10.1016/j.athoracsur.2014.10.070. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.MATHAPATI S, VERMA RS, CHERIAN KM, GUHATHAKURTA S. Inflammatory responses of tissue-engineered xenografts in a clinical scenario. Interact Cardiovasc Thorac Surg. 2011;12:360–365. doi: 10.1510/icvts.2010.256719. [DOI] [PubMed] [Google Scholar]
- 15.WONG ML, GRIFFITHS LG. Immunogenicity in xenogeneic scaffold generation: antigen removal vs. decellularization. Acta Biomater. 2014;10:1806–1816. doi: 10.1016/j.actbio.2014.01.028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.GALILI U. Anti-Gal: an abundant human natural antibody of multiple pathogeneses and clinical benefits. Immunology. 2013;140:1–11. doi: 10.1111/imm.12110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.YURIEV E, AGOSTINO M, FARRUGIA W, et al. Structural biology of carbohydrate xenoantigens. Expert Opin Biol Ther. 2009;9:1017–1029. doi: 10.1517/14712590903066703. [DOI] [PubMed] [Google Scholar]
- 18.KONAKCI KZ, BOHLE B, BLUMER R, et al. Alpha-Gal on bioprostheses: xenograft immune response in cardiac surgery. Eur J Clin Invest. 2005;35:17–23. doi: 10.1111/j.1365-2362.2005.01441.x. [DOI] [PubMed] [Google Scholar]
- 19.NASO F, GANDAGLIA A, BOTTIO T, et al. First quantification of alpha-Gal epitope in current glutaraldehyde-fixed heart valve bioprostheses. Xenotransplantation. 2013;20:252–261. doi: 10.1111/xen.12044. [DOI] [PubMed] [Google Scholar]
- 20.PARK CS, OH SS, KIM YE, et al. Anti-alpha-Gal antibody response following xenogeneic heart valve implantation in adults. J Heart Valve Dis. 2013;22:222–229. [PubMed] [Google Scholar]
- 21.BYRNE GW, MCGREGOR CG. First quantification of alpha-Gal epitope in current glutaraldehyde-fixed heart valve bioprosthesis (by Naso et al.). Xenotransplantation. 2014;21:11–12. doi: 10.1111/xen.12072. [DOI] [PubMed] [Google Scholar]
- 22.MCGREGOR CG, KOGELBERG H, VLASIN M, BYRNE GW. Gal-knockout bioprostheses exhibit less immune stimulation compared to standard biological heart valves. J Heart Valve Dis. 2013;22:383–390. [PubMed] [Google Scholar]
- 23.MIYAGAWA S, YAMAMOTO A, MATSUNAMI K, et al. Complement regulation in the GalT KO era. Xenotransplantation. 2010;17:11–25. doi: 10.1111/j.1399-3089.2010.00569.x. [DOI] [PubMed] [Google Scholar]
- 24.BARONE A, BENKTANDER J, TENEBERG S, BREIMER ME. Characterization of acid and non-acid glycosphingolipids of porcine heart valve cusps as potential immune targets in biological heart valve grafts. Xenotransplantation. 2014;21:510–522. doi: 10.1111/xen.12123. [DOI] [PubMed] [Google Scholar]
- 25.BYRNE GW, STALBOERGER PG, DU Z, DAVIS TR, MCGREGOR CG. Identification of new carbohydrate and membrane protein antigens in cardiac xenotransplantation. Transplantation. 2011;91:287–292. doi: 10.1097/TP.0b013e318203c27d. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.BREIMER ME. Gal/non-Gal antigens in pig tissues and human non-Gal antibodies in the GalT-KO era(1). Xenotransplantation. 2011;18:215–228. doi: 10.1111/j.1399-3089.2011.00644.x. [DOI] [PubMed] [Google Scholar]
- 27.PADLER-KARAVANI V, VARKI A. Potential impact of the non-human sialic acid N-glycolylneuraminic acid on transplant rejection risk. Xenotransplantation. 2011;18:1–5. doi: 10.1111/j.1399-3089.2011.00622.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.SALAMA A, EVANNO G, HARB J, SOULILLOU JP. Potential deleterious role of anti-Neu5Gc antibodies in xenotransplantation. Xenotransplantation. 2015;22:85–94. doi: 10.1111/xen.12142. [DOI] [PubMed] [Google Scholar]
- 29.LEE W, HARA H, COOPER DK, MANJI RA. Expression of NeuGc on pig heart valves. Xenotransplantation. 2015;22:153–154. doi: 10.1111/xen.12162. [DOI] [PubMed] [Google Scholar]
- 30.SAETHRE M, BAUMANN BC, FUNG M, SEEBACH JD, MOLLNES TE. Characterization of natural human anti-non-gal antibodies and their effect on activation of porcine gal-deficient endothelial cells. Transplantation. 2007;84:244–250. doi: 10.1097/01.tp.0000268815.90675.d5. [DOI] [PubMed] [Google Scholar]
- 31.VARKI A. Multiple changes in sialic acid biology during human evolution. Glycoconj J. 2009;26:231–245. doi: 10.1007/s10719-008-9183-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.AMON R, REUVEN EM, LEVIATAN BEN-ARYE S, PADLER-KARAVANI V. Glycans in immune recognition and response. Carbohydr Res. 2014;389:115–122. doi: 10.1016/j.carres.2014.02.004. [DOI] [PubMed] [Google Scholar]
- 33.TANGVORANUNTAKUL P, GAGNEUX P, DIAZ S, et al. Human uptake and incorporation of an immunogenic nonhuman dietary sialic acid. Proc Natl Acad Sci U S A. 2003;100:12045–12050. doi: 10.1073/pnas.2131556100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.PADLER-KARAVANI V, YU H, CAO H, et al. Diversity in specificity, abundance, and composition of anti-Neu5Gc antibodies in normal humans: potential implications for disease. Glycobiology. 2008;18:818–830. doi: 10.1093/glycob/cwn072. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.PHAM T, GREGG CJ, KARP F, et al. Evidence for a novel human-specific xeno-auto-antibody response against vascular endothelium. Blood. 2009;114:5225–5235. doi: 10.1182/blood-2009-05-220400. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.HEDLUND M, PADLER-KARAVANI V, VARKI NM, VARKI A. Evidence for a human-specific mechanism for diet and antibody-mediated inflammation in carcinoma progression. Proc Natl Acad Sci U S A. 2008;105:18936–18941. doi: 10.1073/pnas.0803943105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.SAMRAJ AN, PEARCE OM, LÄUBLI H, et al. A red meat-derived glycan promotes inflammation and cancer progression. Proc Natl Acad Sci U S A. 2015;112:542–547. doi: 10.1073/pnas.1417508112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.SCOBIE L, PADLER-KARAVANI V, LE BAS-BERNARDET S, et al. Long-term IgG response to porcine Neu5Gc antigens without transmission of PERV in burn patients treated with porcine skin xenografts. J Immunol. 2013;191:2907–2915. doi: 10.4049/jimmunol.1301195. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.JEONG HJ, ADHYA M, PARK HM, KIM YG, KIM BG. Detection of Hanganutziu-Deicher antigens in O-glycans from pig heart tissues by matrix-assisted laser desorption/ionization time-of-flight mass spectrometry. Xenotransplantation. 2013;20:407–417. doi: 10.1111/xen.12045. [DOI] [PubMed] [Google Scholar]
- 40.DIAZ SL, PADLER-KARAVANI V, GHADERI D, et al. Sensitive and specific detection of the non-human sialic Acid N-glycolylneuraminic acid in human tissues and biotherapeutic products. PLoS ONE. 2009;4:e4241. doi: 10.1371/journal.pone.0004241. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.HARA S, YAMAGUCHI M, TAKEMORI Y, et al. Determination of mono-O-acetylated N-acetylneuraminic acids in human and rat sera by fluorometric high-performance liquid chromatography. Anal Biochem. 1989;179:162–166. doi: 10.1016/0003-2697(89)90218-2. [DOI] [PubMed] [Google Scholar]
- 42.PADLER-KARAVANI V, SONG X, YU H, et al. Cross-comparison of protein recognition of sialic acid diversity on two novel sialoglycan microarrays. J Biol Chem. 2012;287:22593–22608. doi: 10.1074/jbc.M112.359323. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.MANJI RA, ZHU LF, NIJJAR NK, et al. Glutaraldehyde-fixed bioprosthetic heart valve conduits calcify and fail from xenograft rejection. Circulation. 2006;114:318–327. doi: 10.1161/CIRCULATIONAHA.105.549311. [DOI] [PubMed] [Google Scholar]
- 44.PIBAROT P, DUMESNIL JG. Prosthetic heart valves: selection of the optimal prosthesis and long-term management. Circulation. 2009;119:1034–1048. doi: 10.1161/CIRCULATIONAHA.108.778886. [DOI] [PubMed] [Google Scholar]
- 45.VADORI M, COZZI E. Immunological challenges and therapies in xenotransplantation. Cold Spring Harb Perspect Med. 2014;4:a015578. doi: 10.1101/cshperspect.a015578. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.CHRISTIANSEN D, MOUHTOURIS E, RAMSLAND PA, SANDRIN MS. Studies on carbohydrate xenoantigens. Methods Mol Biol. 2012;885:47–56. doi: 10.1007/978-1-61779-845-0_4. [DOI] [PubMed] [Google Scholar]
- 47.GALILI U. Induced anti-non gal antibodies in human xenograft recipients. Transplantation. 2012;93:11–16. doi: 10.1097/TP.0b013e31823be870. [DOI] [PubMed] [Google Scholar]
- 48.HUMAN P, ZILLA P. Inflammatory and immune processes: the neglected villain of bioprosthetic degeneration. J Long Term Eff Med Implants. 2001;11:199–220. [PubMed] [Google Scholar]
- 49.MCGREGOR CG, CARPENTIER A, LILA N, LOGAN JS, BYRNE GW. Cardiac xenotransplantation technology provides materials for improved bioprosthetic heart valves. J Thorac Cardiovasc Surg. 2011;141:269–275. doi: 10.1016/j.jtcvs.2010.08.064. [DOI] [PubMed] [Google Scholar]
- 50.LUTZ AJ, LI P, ESTRADA JL, et al. Double knockout pigs deficient in N-glycolylneuraminic acid and galactose alpha-1,3-galactose reduce the humoral barrier to xenotransplantation. Xenotransplantation. 2013;20:27–35. doi: 10.1111/xen.12019. [DOI] [PubMed] [Google Scholar]