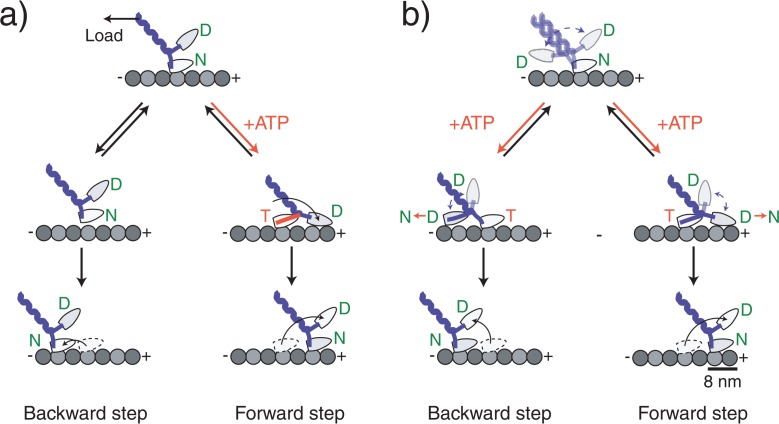
Figure 5.
Molecular models of kinesin stepping. Kinesin moves in a walking manner using its two head domains. (a) A model in which the backward steps is not coupled with ATP binding. ATP binding to the nucleotide-free head that is attached directly to the microtubule coerces a forward-directed mechanical change in the neck domain forcing the partner head to the forward direction. After the detached head has landed, the alternate head releases from the microtubule as a result of an internal strain between the heads completing the forward step. Backward steps occur because load causes the head to briefly detach, but it quickly reattaches at a nearby backward site. (b) A model in which the backward step is coupled with ATP binding. ATP binding to the nucleotide-free head does not make an ATPase-coupled mechanical change. Instead it relieves the inhibition which allows the partner head to search for adjacent binding sites on the microtubule by undergoing a microtubule-activated ADP release of the partner head. The direction of the step is biased to the forward direction by an asymmetric free energy landscape such as a rachet-like structure or an asymmetric steric effect. The originally bound head releases after the detached head attaches. Letters in the circle at the head represent the prospective binding nucleotide: T=ATP, D=ADP, Pi=phosphate.