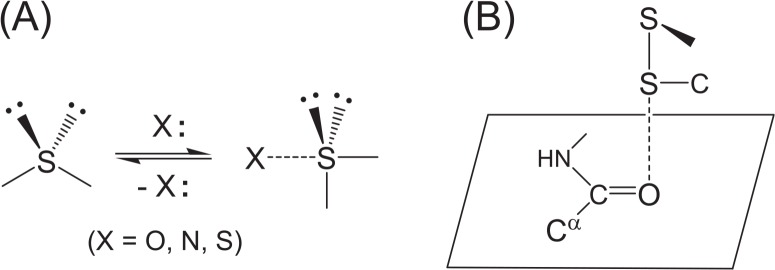
Figure 1.
Formation and structural features of the S···X interaction. (A) The divalent S atom adopts the coordination from a heteroatom X, changing the valence state from a tetrahedral to a hypervalent trigonal bipyramidal state. (B) The most frequently observed S···O interactions in proteins. The S atom of a disulfide bond (SSC type) approaches a main-chain amide O atom in a direction vertical to the peptide plane maintaining the linearity of the three S–S···O atoms.