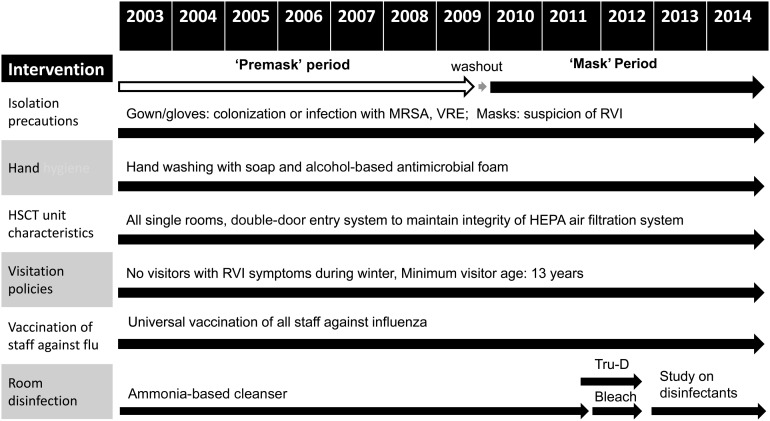
Figure 1.
Infection control practices during “premask” and “postmask” period are as follows: (1) isolation precautions including use of gown and gloves when patients have or are colonized with methicillin-resistant Staphylococcus aureus (MRSA) and vancomycin-resistant Enterococcus (VRE), and use of gowns, gloves, and surgical masks if a patient has symptoms of an upper respiratory tract infection; (2) hand washing, which includes both washing with soap before entering the outpatient or inpatient hematopoietic stem cell transplant (HSCT) units as well as additional hand washing or hand sanitization with an antibacterial alcohol-based foam before patient contact (of note, a hospital-wide campaign for aggressive hand hygiene started in April 2009, although compliance in the bone marrow transplant unit has consistently been high, ie, 96% average [range, 94%–98%]); (3) all single rooms and double-door entry systems to maintain the integrity of the high-efficiency particulate air (HEPA) filtration system for the HSCT inpatient unit; in the outpatient unit/day hospital, patients are either seen in individual rooms if positive or suspected to have a communicable organism (eg, MRSA, VRE, respiratory viral infection [RVI]) or 1 of 2 open treatment areas separated by curtains; (4) visitation policies limiting visitors with RVI symptoms; (5) universal vaccination of staff against influenza; and (6) disinfection protocols with ammonia or bleach with or without Tru-D ultraviolet-C room disinfection. Of note, while the HSCT unit switched from ammonia to bleach in March 2011, further changes took place between April 2012 and August 2014 as part of a hospital-wide study examining room disinfection protocols for contact isolation rooms: ammonia was used from April 2012 to October 2012; bleach from November 2012 to May 2013; ammonia again from June 2013 to December 2013; and bleach again from January 2014 to August 2014 (however, rooms of patients with Clostridium difficile were always cleaned with bleach). The HSCT unit also began using Tru-D in February 2011; as part of the above study, hospital-wide use of this machine was also regulated from April 2012 to August 2014 as follows: Tru-D was used from April 2012 to October 2012, not used November 2012 to December 2013, and used again from January 2014 to July 2014.