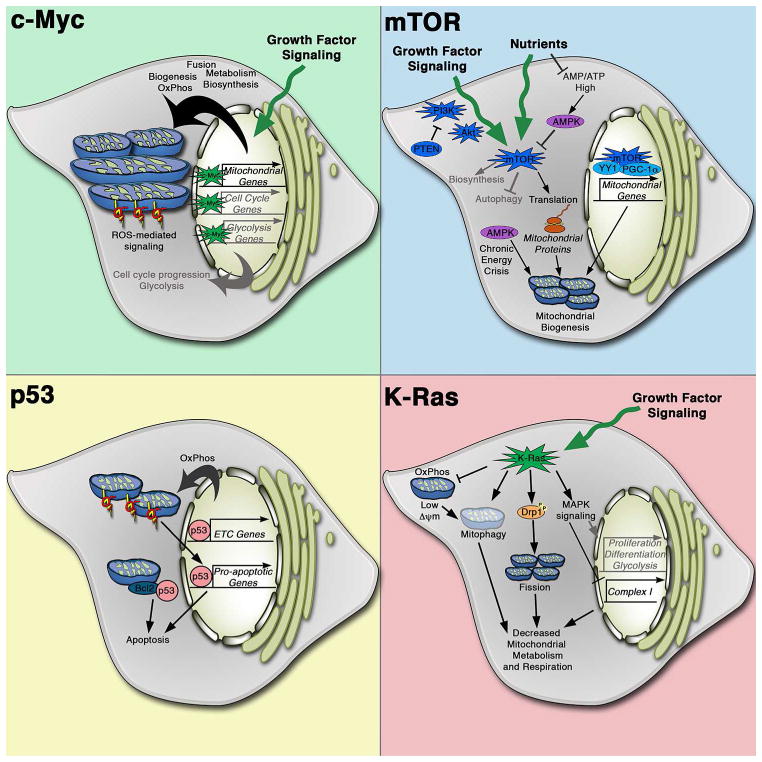
Figure 3. Effects of classical oncogenic and tumor suppressive pathways on mitochondrial biology.
Key mechanisms of mitochondrial regulation by c-MYC, K-RAS, PI3K and p53 signaling pathways. Through transcriptional regulation, c-Myc induces mitochondrial biogenesis and metabolism in addition to its stimulation of cell cycle progression and glycolysis. c-Myc promotes mitochondrial fusion and respiration, which can result in increased ROS production and oxidative signaling. Hyperactive PI3K signaling through either PI3K mutation or loss/mutatoin of the PTEN tumor suppressor results in mTOR activation, which is additionally regulated by nutrient availability, to regulate cell growth. mTOR promotes mitochondrial biogenesis both transcriptionally and translationally. Low nutrient conditions that result in a high AMP/ATP ratio result in AMPK activation, which opposes the mTOR pathway. During chronic nutrient deprivation, AMPK can also promote mitochondrial biogenesis to allow for metabolic flexibility. Loss of p53 promotes survival not only via transcriptional regulation of cell death programs but also through direct interactions with Bcl-2 proteins at the mitochondria. p53 can also induce mitochondrial respiration to promote tumorigenesis by allowing for metabolic flexibility. Oncogenic K-Ras mutations result in a coordinated program of mitochondrial regulation, inhibiting respiration through multiple mechanisms as well as promoting mitochondrial fission and mitophagy.