Abstract
Sialoadhesin is a nonphagocytic lectin-like receptor found on a restricted population of tissue macrophages in lymphoid and hemopoietic tissues. In bone marrow, it is localized to areas of contact between the resident stromal macrophages and developing granulocytes, which together form myeloblastic clusters. Sialoadhesin is highly specific for sialylated glycoconjugates and may play a role in adhesion and trophic hemopoietic cell interactions, although its function is unknown. Resident peritoneal macrophages do not express high levels of sialoadhesin in vitro unless an inducing element found in normal mouse serum is present. The restricted in vivo location of this marker and its induction by mouse serum prompted us to examine the possible influence of various cytokines on its expression, measured by a sheep erythrocyte rosetting assay. None of the cytokines tested was able to induce sialoadhesin; however, interleukin 4 (IL-4) prevented the induction in the presence of serum. Expression of other macrophage markers was not influenced in parallel, and Western blotting showed that sialoadhesin antigen in cell lysates was selectively reduced by IL-4. Inhibition by IL-4 was dose dependent, could be blocked by antibodies to both IL-4 and the IL-4 receptor, and was overcome by increased serum concentrations. IL-4 is therefore a potent cytokine regulator of the sialic acid-specific receptor implicated in macrophage-hemopoietic cell interactions.
Full text
PDF
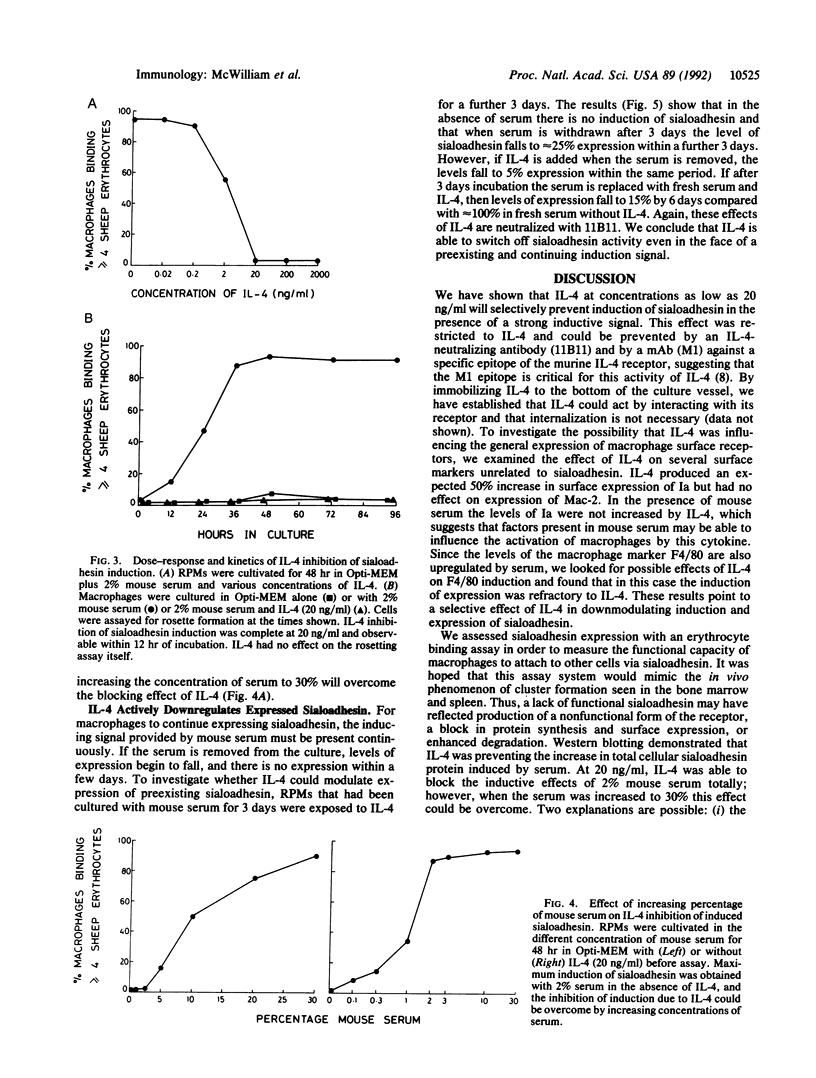
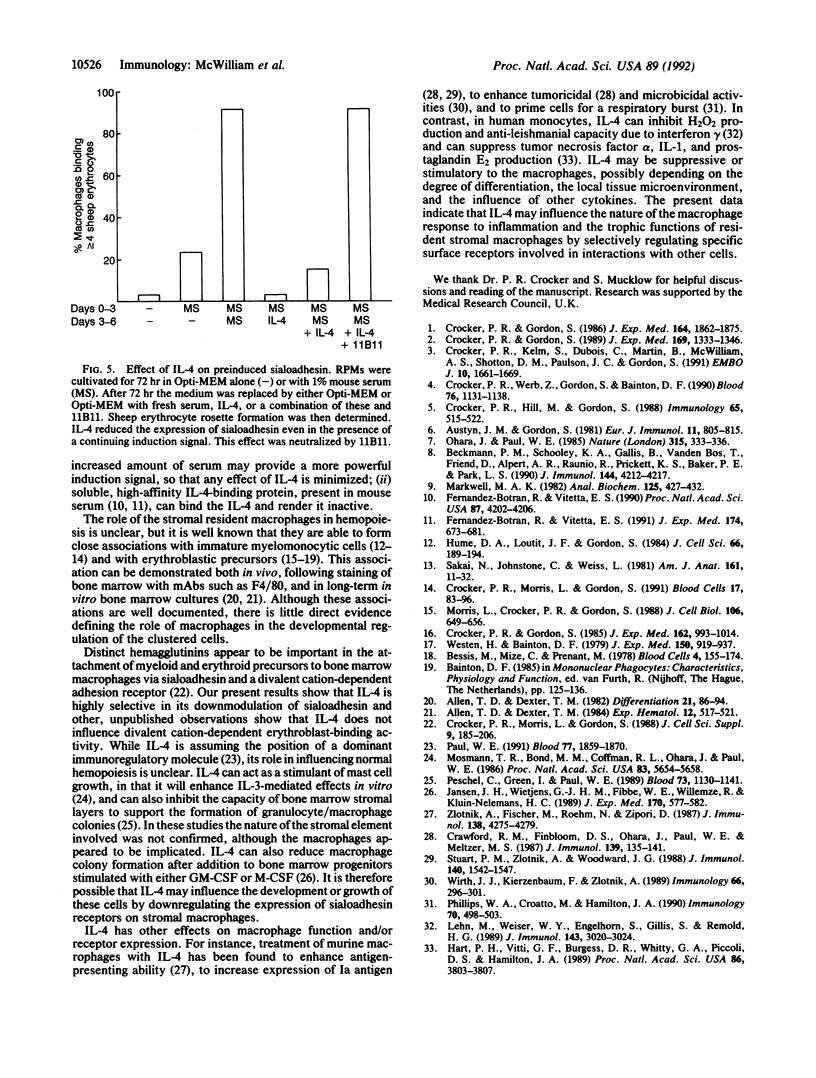
Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Allen T. D., Dexter T. M. The essential cells of the hemopoietic microenvironment. Exp Hematol. 1984 Aug;12(7):517–521. [PubMed] [Google Scholar]
- Allen T. D., Dexter T. M. Ultrastructural aspects of erythropoietic differentiation in long-term bone marrow culture. Differentiation. 1982;21(2):86–94. doi: 10.1111/j.1432-0436.1982.tb01201.x. [DOI] [PubMed] [Google Scholar]
- Austyn J. M., Gordon S. F4/80, a monoclonal antibody directed specifically against the mouse macrophage. Eur J Immunol. 1981 Oct;11(10):805–815. doi: 10.1002/eji.1830111013. [DOI] [PubMed] [Google Scholar]
- Beckmann M. P., Schooley K. A., Gallis B., Vanden Bos T., Friend D., Alpert A. R., Raunio R., Prickett K. S., Baker P. E., Park L. S. Monoclonal antibodies block murine IL-4 receptor function. J Immunol. 1990 Jun 1;144(11):4212–4217. [PubMed] [Google Scholar]
- Bessis M., Mize C., Prenant M. Erythropoiesis: comparison of in vivo and in vitro amplification. Blood Cells. 1978;4(1-2):155–174. [PubMed] [Google Scholar]
- Crawford R. M., Finbloom D. S., Ohara J., Paul W. E., Meltzer M. S. B cell stimulatory factor-1 (interleukin 4) activates macrophages for increased tumoricidal activity and expression of Ia antigens. J Immunol. 1987 Jul 1;139(1):135–141. [PubMed] [Google Scholar]
- Crocker P. R., Gordon S. Isolation and characterization of resident stromal macrophages and hematopoietic cell clusters from mouse bone marrow. J Exp Med. 1985 Sep 1;162(3):993–1014. doi: 10.1084/jem.162.3.993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Crocker P. R., Gordon S. Mouse macrophage hemagglutinin (sheep erythrocyte receptor) with specificity for sialylated glycoconjugates characterized by a monoclonal antibody. J Exp Med. 1989 Apr 1;169(4):1333–1346. doi: 10.1084/jem.169.4.1333. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Crocker P. R., Gordon S. Properties and distribution of a lectin-like hemagglutinin differentially expressed by murine stromal tissue macrophages. J Exp Med. 1986 Dec 1;164(6):1862–1875. doi: 10.1084/jem.164.6.1862. [DOI] [PMC free article] [PubMed] [Google Scholar]
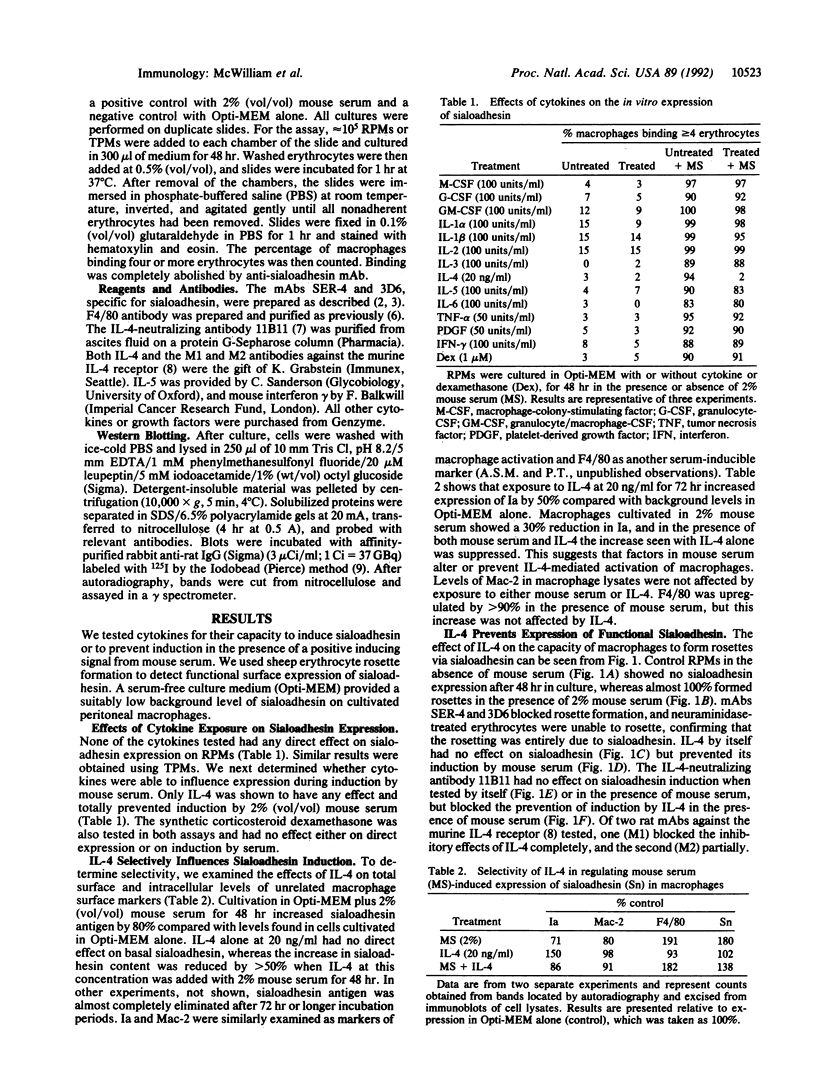
- Crocker P. R., Hill M., Gordon S. Regulation of a murine macrophage haemagglutinin (sheep erythrocyte receptor) by a species-restricted serum factor. Immunology. 1988 Dec;65(4):515–522. [PMC free article] [PubMed] [Google Scholar]
- Crocker P. R., Kelm S., Dubois C., Martin B., McWilliam A. S., Shotton D. M., Paulson J. C., Gordon S. Purification and properties of sialoadhesin, a sialic acid-binding receptor of murine tissue macrophages. EMBO J. 1991 Jul;10(7):1661–1669. doi: 10.1002/j.1460-2075.1991.tb07689.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Crocker P. R., Morris L., Gordon S. Adhesion receptors involved in the erythroblastic island. Blood Cells. 1991;17(1):83–96. [PubMed] [Google Scholar]
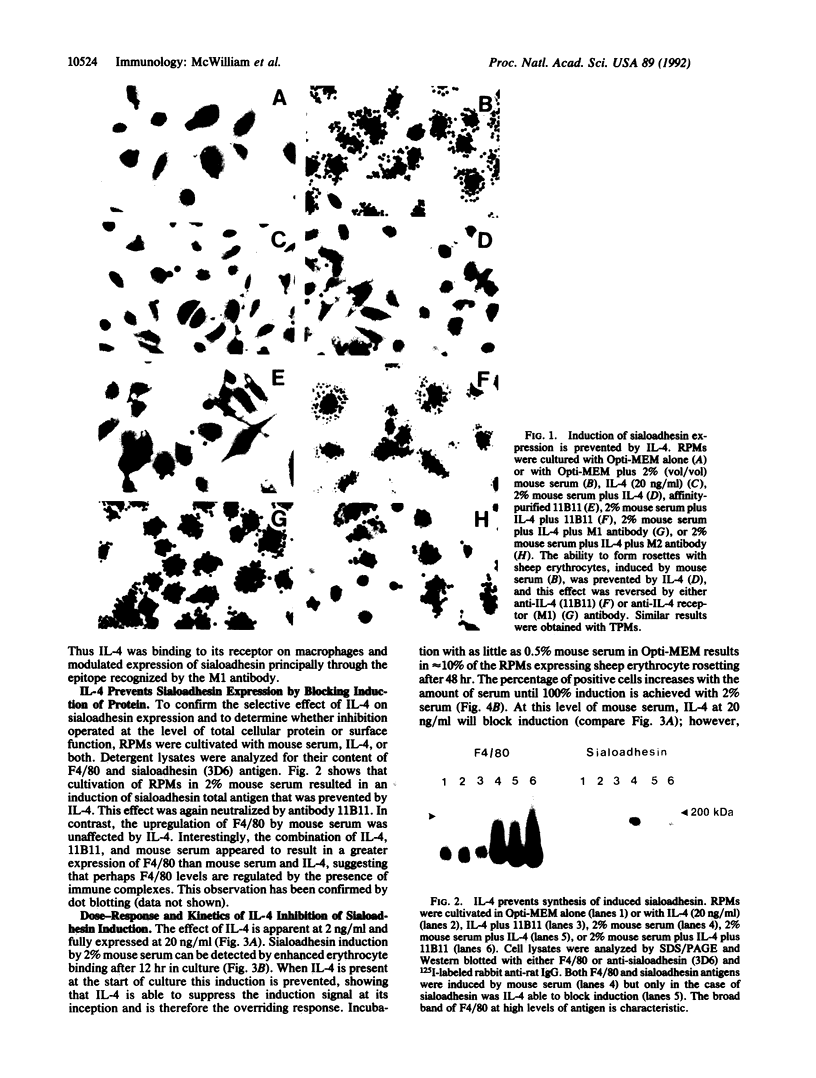
- Crocker P. R., Morris L., Gordon S. Novel cell surface adhesion receptors involved in interactions between stromal macrophages and haematopoietic cells. J Cell Sci Suppl. 1988;9:185–206. doi: 10.1242/jcs.1988.supplement_9.10. [DOI] [PubMed] [Google Scholar]
- Crocker P. R., Werb Z., Gordon S., Bainton D. F. Ultrastructural localization of a macrophage-restricted sialic acid binding hemagglutinin, SER, in macrophage-hematopoietic cell clusters. Blood. 1990 Sep 15;76(6):1131–1138. [PubMed] [Google Scholar]
- Fernandez-Botran R., Vitetta E. S. A soluble, high-affinity, interleukin-4-binding protein is present in the biological fluids of mice. Proc Natl Acad Sci U S A. 1990 Jun;87(11):4202–4206. doi: 10.1073/pnas.87.11.4202. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fernandez-Botran R., Vitetta E. S. Evidence that natural murine soluble interleukin 4 receptors may act as transport proteins. J Exp Med. 1991 Sep 1;174(3):673–681. doi: 10.1084/jem.174.3.673. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hart P. H., Vitti G. F., Burgess D. R., Whitty G. A., Piccoli D. S., Hamilton J. A. Potential antiinflammatory effects of interleukin 4: suppression of human monocyte tumor necrosis factor alpha, interleukin 1, and prostaglandin E2. Proc Natl Acad Sci U S A. 1989 May;86(10):3803–3807. doi: 10.1073/pnas.86.10.3803. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hume D. A., Loutit J. F., Gordon S. The mononuclear phagocyte system of the mouse defined by immunohistochemical localization of antigen F4/80: macrophages of bone and associated connective tissue. J Cell Sci. 1984 Mar;66:189–194. doi: 10.1242/jcs.66.1.189. [DOI] [PubMed] [Google Scholar]
- Jansen J. H., Wientjens G. J., Fibbe W. E., Willemze R., Kluin-Nelemans H. C. Inhibition of human macrophage colony formation by interleukin 4. J Exp Med. 1989 Aug 1;170(2):577–582. doi: 10.1084/jem.170.2.577. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lehn M., Weiser W. Y., Engelhorn S., Gillis S., Remold H. G. IL-4 inhibits H2O2 production and antileishmanial capacity of human cultured monocytes mediated by IFN-gamma. J Immunol. 1989 Nov 1;143(9):3020–3024. [PubMed] [Google Scholar]
- Markwell M. A. A new solid-state reagent to iodinate proteins. I. Conditions for the efficient labeling of antiserum. Anal Biochem. 1982 Sep 15;125(2):427–432. doi: 10.1016/0003-2697(82)90025-2. [DOI] [PubMed] [Google Scholar]
- Morris L., Crocker P. R., Gordon S. Murine fetal liver macrophages bind developing erythroblasts by a divalent cation-dependent hemagglutinin. J Cell Biol. 1988 Mar;106(3):649–656. doi: 10.1083/jcb.106.3.649. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mosmann T. R., Bond M. W., Coffman R. L., Ohara J., Paul W. E. T-cell and mast cell lines respond to B-cell stimulatory factor 1. Proc Natl Acad Sci U S A. 1986 Aug;83(15):5654–5658. doi: 10.1073/pnas.83.15.5654. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ohara J., Paul W. E. Production of a monoclonal antibody to and molecular characterization of B-cell stimulatory factor-1. Nature. 1985 May 23;315(6017):333–336. doi: 10.1038/315333a0. [DOI] [PubMed] [Google Scholar]
- Paul W. E. Interleukin-4: a prototypic immunoregulatory lymphokine. Blood. 1991 May 1;77(9):1859–1870. [PubMed] [Google Scholar]
- Peschel C., Green I., Paul W. E. Interleukin-4 induces a substance in bone marrow stromal cells that reversibly inhibits factor-dependent and factor-independent cell proliferation. Blood. 1989 Apr;73(5):1130–1141. [PubMed] [Google Scholar]
- Phillips W. A., Croatto M., Hamilton J. A. Priming the macrophage respiratory burst with IL-4: enhancement with TNF-alpha but inhibition by IFN-gamma. Immunology. 1990 Aug;70(4):498–503. [PMC free article] [PubMed] [Google Scholar]
- Sakai N., Johnstone C., Weiss L. Bone marrow cells associated with heightened eosinophilopoiesis: an electron microscope study of murine bone marrow stimulated by Ascaris suum. Am J Anat. 1981 May;161(1):11–32. doi: 10.1002/aja.1001610103. [DOI] [PubMed] [Google Scholar]
- Stuart P. M., Zlotnik A., Woodward J. G. Induction of class I and class II MHC antigen expression on murine bone marrow-derived macrophages by IL-4 (B cell stimulatory factor 1). J Immunol. 1988 Mar 1;140(5):1542–1547. [PubMed] [Google Scholar]
- Westen H., Bainton D. F. Association of alkaline-phosphatase-positive reticulum cells in bone marrow with granulocytic precursors. J Exp Med. 1979 Oct 1;150(4):919–937. doi: 10.1084/jem.150.4.919. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wirth J. J., Kierszenbaum F., Zlotnik A. Effects of IL-4 on macrophage functions: increased uptake and killing of a protozoan parasite (Trypanosoma cruzi). Immunology. 1989 Feb;66(2):296–301. [PMC free article] [PubMed] [Google Scholar]
- Zlotnik A., Fischer M., Roehm N., Zipori D. Evidence for effects of interleukin 4 (B cell stimulatory factor 1) on macrophages: enhancement of antigen presenting ability of bone marrow-derived macrophages. J Immunol. 1987 Jun 15;138(12):4275–4279. [PubMed] [Google Scholar]