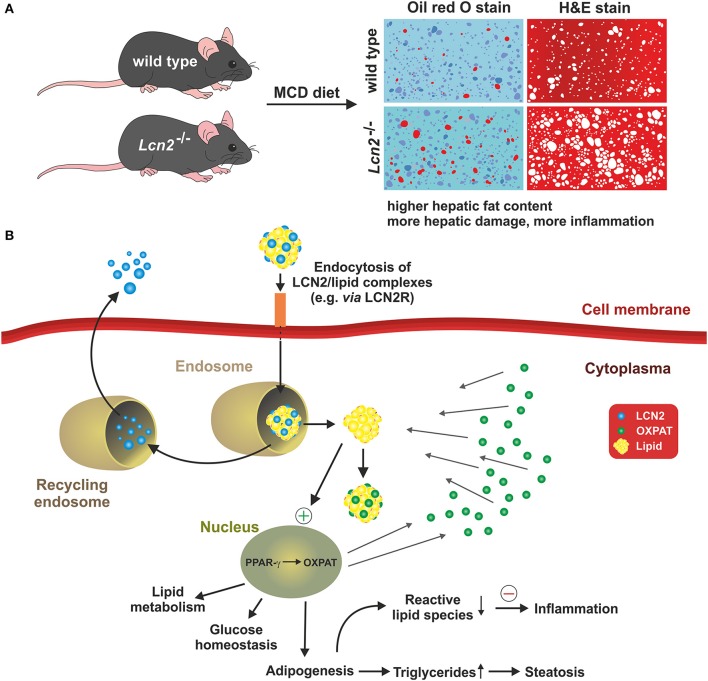
Figure 4.
LCN2 and fat metabolism. A recent concept suggests that LCN2 is a key factor in controlling intracellular fat metabolisms in hepatocytes by regulating expression of the lipid droplet protein PLIN5/OXPAT. (A) The concept is majorly based on the finding that mice lacking LCN2 accumulate more lipids in the liver and show more hepatic damage and inflammation when fed a methionine-choline deficient diet (MCD) representing a nutritional model of NASH. (B) In the respective study, it was proposed that LCN2 imports lipids into hepatocytes either via specific receptors (e.g., LCN2R) or unknown endocytosis pathways. Within the cytoplasm, the LCN2/lipid complexes are first packed into endosomes whose slightly acidified microenvironment causes LCN2 to dissociate from the lipids. The lipids then move into the cytoplasm, where they are coated by PLIN5/OXPAT protecting them from intracellular degradation and oxidation. LCN2 may be recycled and secreted. PLIN5/OXPAT itself is up-regulated by PPAR-γ that is stimulated by the higher cytoplasmic fat content that is the consequence of facilitated lipid import by LCN2. Together, the suspected interaction of LCN2, PPAR-γ, and PLIN5/OXPAT presents a complex network that affects lipid metabolism, glucose homeostasis, and adipogenesis. In the presence of LCN2, intracellular concentrations of free reactive lipid species may be reduced and the overall inflammatory responses suppressed. Contrarily, in the absence of LCN2, the concentration of free fatty acids within the cytosol is increased predisposing for inflammation and steatosis.