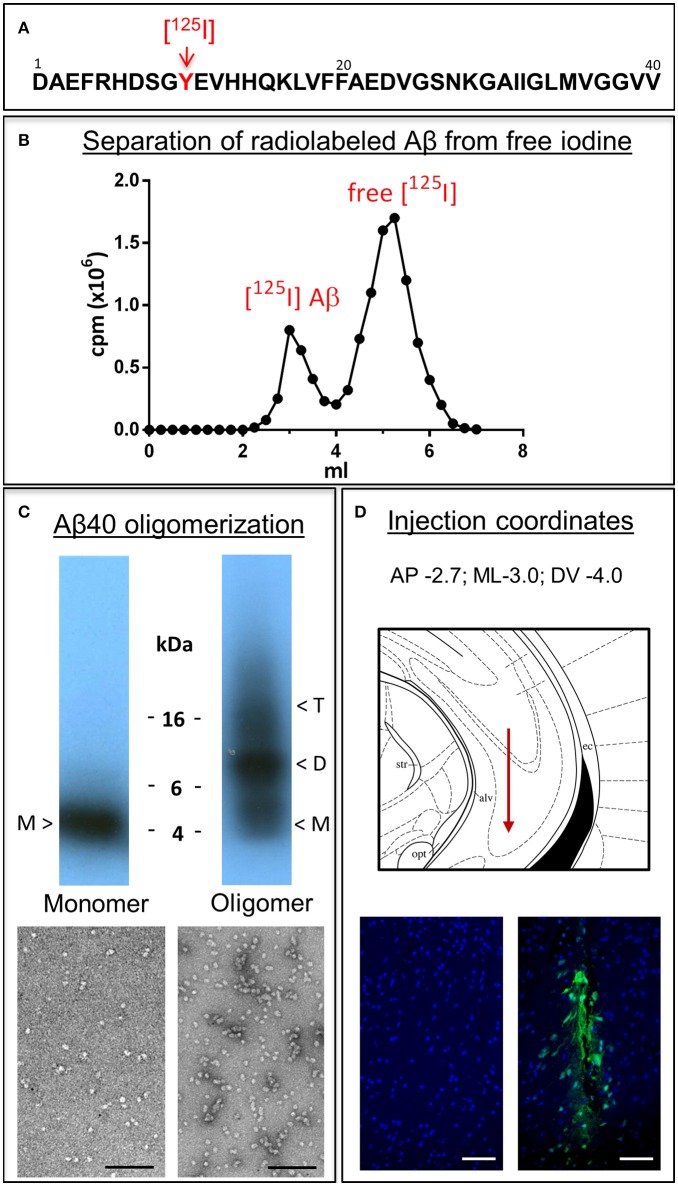
Figure 1.
Aβ1-40 radioiodination, assessment of peptide oligomerization and intra-cerebral injection. (A) Amino acid sequence of Aβ1-40 highlighting the location of tyrosine 10, the target of the radioiodination procedure (red arrow). (B) Representative separation of [125I]-labeled Aβ1-40 from free iodine using a desalting 1.8 kDa cut-off polyacrylamide column. (C) Autoradiogram following electrophoretic separation of monomeric and oligomeric preparations of radiolabeled Aβ1-40 on 16.5% SDS-polyacrylamide gels (top) and EM images illustrating the differential conformational assemblies negatively stained with uranyl acetate (bottom). Magnification: bar represents 100 nm. (D) Schematic representation of the needle location for the intra-cerebral injection of Aβ1-40 preparations (top panel) and immunostaining with monoclonal anti-Aβ 6E10 followed by Alexafluor 488 conjugated secondary antibody and DAPI counterstain at the injection site demonstrating minimal—although unavoidable—tissue disruption (bottom right panel). The absence of Aβ signal in the contralateral site in animals sacrificed immediately after Aβ injection corroborates the specificity of the immunostaining (bottom left panel). Magnification: bar represents 100 μm.