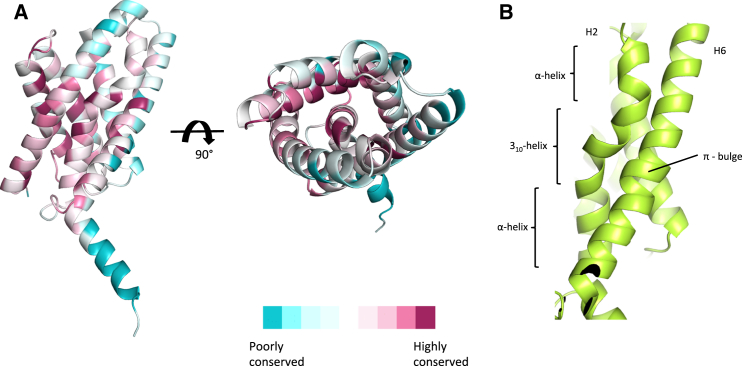
Figure 3.
Structural Overview of the Conserved Motifs in the Group I S Component YkoE
(A) Substrate cavity and interhelical contacts are the most conserved regions of YkoE. Conservation of amino acid residues was analyzed using ConSurf (Ashkenazy et al., 2010). 980 non-redundant sequences of YkoE homologs were used in the alignment to emphasize the most conserved regions of the structure. Highly conserved residues are depicted as burgundy patches; moderately conserved side chains are shown in light pink. Weakly conserved residues are colored in cyan. Residues that exhibit some degree of conservation among the 980 homologs of YkoE are in white.
(B) Ribbon representation of the packing conformations of helix H2 and helix H6. Helix H2 is comprised of α-310-α helical elements that allow it to pack tightly against helix H6, thereby closing the cavity from the cytoplasmic side. The highly conserved π bulge is in the middle of helix H6.