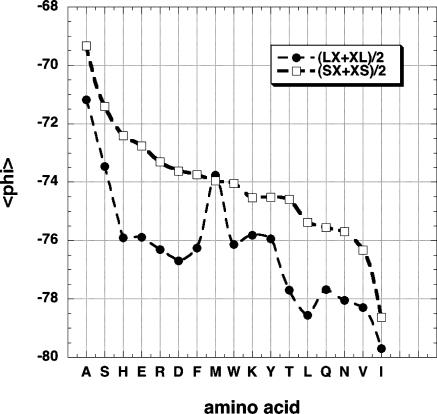
Fig. 4.
The neighboring residue effect seen by plotting 〈φ〉 for the central residues of tripeptides excised from the coil library; the neighboring residues are either L or S. Only backbone conformations contained in the PPII window (–100° > φ > –40°, 90° < ψ < 180°) are counted. The average of the values found for preceding (LX or SX) and following (XL or XS) neighbors is shown. The amino acids are ordered by the 〈φ〉 values of residues with S neighbors.