Abstract
Cell migration is an essential process in organism development and physiological maintenance. Although current methods permit accurate comparisons of the effects of molecular manipulations and drug applications on cell motility, effects of alterations in subcellular activities on motility cannot be fully elucidated from those methods. Here, we develop a strategy termed cell-nuclear (CN) correlation to parameterize represented dynamic subcellular activities and to quantify their contributions in mesenchymal-like migration. Based on the biophysical meaning of the CN correlation, we propose a cell migration potential index (CMPI) to measure cell motility. When the effectiveness of CMPI was evaluated with respect to one of the most popular cell migration analysis methods, Persistent Random Walk, we found that the cell motility estimates among six cell lines used in this study were highly consistent between these two approaches. Further evaluations indicated that CMPI can be determined using a shorter time period and smaller cell sample size, and it possesses excellent reliability and applicability, even in the presence of a wide range of noise, as might be generated from individual imaging acquisition systems. The novel approach outlined here introduces a robust strategy through an analysis of subcellular locomotion activities for single cell migration assessment.
Cell migration is a highly coordinated event that plays a central role in a broad range of physiological and pathological events, including embryonic development1,2, wound healing3,4,5 and cancer metastasis6,7,8,9. During organogenesis, cell migration is critical to sustaining the functions of organ systems. Failure of cell migration to the correct positions can have grave consequences. For example, defective cell migration of the cardiac neural crest cells impairs aorticopulmonary septation in Splotch mutant mice and is often fatal10. Clinically, cell migration is particularly relevant as among cancer patients, the 5-year mortality rate is dramatically increased if cancer cells become metastatic, i.e., inappropriately escaping from their original location and migrating to distal sites9,11. Hence, the ability to precisely assess cell migration states could provide valuable insight into cell physiology and pathology.
Currently, the primary tools for quantitatively assessing the cell migration capacity are the wound-healing assay and single cell trajectory analysis. The wound-healing assay mimics the healing process of epithelial tissue12,13. It measures the ability of a monolayer of confluent cells to migrate into and fully cover a denuded area (a wound), which is created by scraping cells out of the monolayer. Effects of disruptive factors such as extracellular stimuli (drugs) or intracellular alterations (protein expression or activity changes) can be assessed by measuring their capacity to modulate the rate of confluence restoration14,15. Cell trajectory analysis of single mesenchymal-like cells is performed through using mathematical models, mainly persistent random walk (PRW)16. In this model, cell speed and average persistence time of duration (i.e., persistence time) as a cell moves towards or away from a stimulus are the critical parameters of cell motility.
These two approaches treat cells as a simple, uniform object and deal only with the positions and the trajectories of the cells12,14,16,17. Hence, variations among cells in migration strategies that result in different inherent migration capacities cannot be revealed directly from these approaches. In mesenchymal-like cells, cell migration involves complicated subcellular processes18,19,20. However, all these processes can be classified into two common, independent subcellular activities. The first is the formation of lamellipodia and filopodia in the cell leading edge, which enables a cell to protrude and establish new contacts with its environment21. The second is actomyosin contraction, which induces tension through stress fibers and induces the detachment of contact points at the trailing edge of the cell and drags the cell body toward the leading edge22. The detailed molecular mechanisms of these two subcellular activities have been elucidated23,24,25,26,27; yet, this knowledge has not resulted in a complete understanding of the relationship between mesenchymal-like cell migration patterns and cell motility.
Here, we report a new approach to evaluating cell motility through the contributions of these two common subcellular activities. The nucleus is tightly connected to the cytoskeleton28 and is responsive to cell movement29; therefore, it is possible to assess the cell migration status by analyzing the motion of the cell relative to the nucleus. We correlated individual cell centroid displacements with nuclear centroid displacements to identify real-time subcellular activities during cell migration. Consequently, we evaluated mesenchymal-like cell motility by concurrent analysis of the contributions of these two subcellular activities. Through cell movement analysis, this work provides a biological and physical connection between migration potential and molecular mechanisms of mesenchymal-like cell migration.
Results
CN correlations
Mesenchymal-like cell migration is a discrete process that can be classified into two main types of independent subcellular events: leading edge protrusions and trailing edge detachments. In a mesenchymal-like cell, the nucleus is generally located at the trailing edge. When a cell extends its leading edge to probe the environment, the nucleus remains stationary. Once the cell establishes new contacts and determines where to move, actomyosin contractions occur in the trailing edge; local adhesion sites are detached and the nucleus moves coherently with the main body of the cell. Since leading edge protrusions and trailing-edge detachments are distinguishable by the relative motion between momentary cell centroid displacement (CCD) and coupled nucleus centroid displacement (NCD). The relation between the CCD and the NCD might be applied to analyze cell migration.
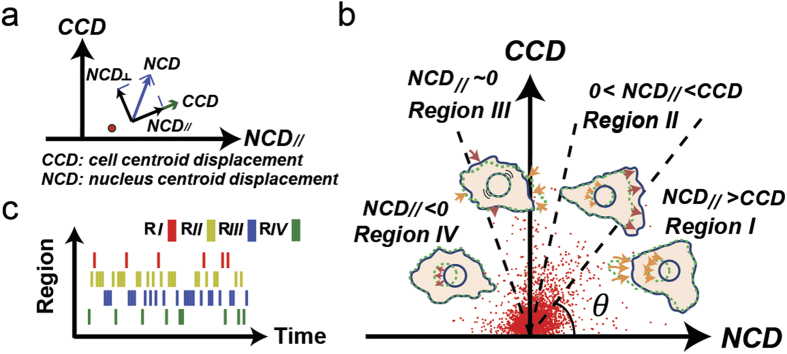
When a correlation exists between a CCD and coupled NCD, this correlation should share a common driving force. Hence, when the correlation is analyzed, the transient direction of every CCD could be set as the reference direction to correlate to the coupled NCD projected in the same direction (denoted as NCD//) (Fig. 1a). Based on the mesenchymal migratory mechanism, a trailing edge detachment event should yield a significant NCD// due to the coherent movements between the cell body and the nucleus. In contrast, in a leading edge protrusion event, the NCD// should be negligible because during the event the nucleus remains relatively stationary. We term the correlation between a CCD and the concomitant NCD// a CN correlation and describe the migration status of a cell through a collection of CN correlations.
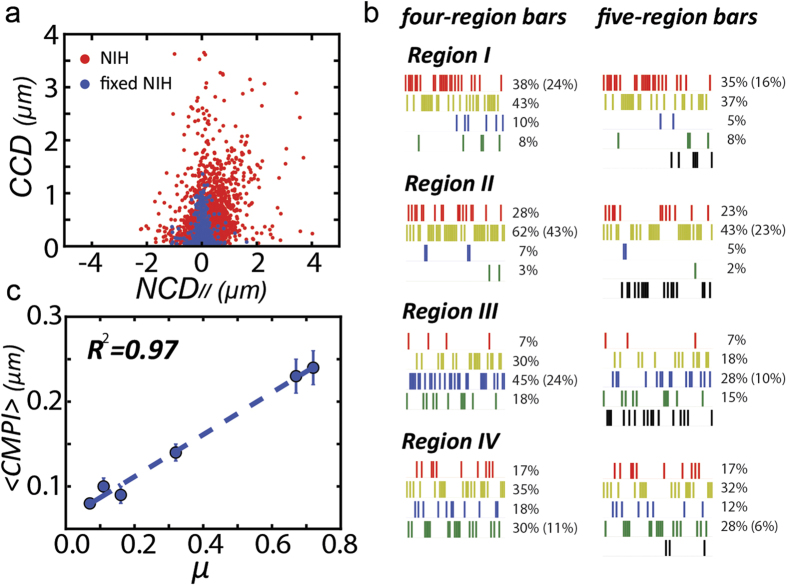
Figure 1. Correlations between cell and nucleus displacements can describe subcellular activities of cell migration.
(a) The CN correlation data point (red dot) locates the correlation between a CCD and a coupled NCD// (projection of nucleus centroid displacement in the CCD direction) in a CCD vs. NCD// plot. (b) Four separate regions (separated by dash lines at 45°, 75°, and 105°) in the CCD-NCD// coordinate system correspond to distinct subcellular migratory activities. A cluster of red dots shown are the CN correlation data acquired from 50 NIH 3T3 fibroblasts tracked at 1-min. time intervals for 1 hour. (c) The barcode enables visualization of the sequential occurrence of CN correlations in the four regions. Regions are distinguished by red, yellow, blue, and green, respectively. Each bar represents a CN correlation data point acquired at a 1-min time interval.
CN correlation analysis is a new approach to elucidate the subcellular activities of cell migration
We hypothesize that a CCD-NCD// coordinate system combined with the CN correlation approach can be used to describe the subcellular events of cell migration. The location of a CN correlation data point can be expressed using the polar coordinate system and a range of coordinate angles are used to delineate corresponding subcellular events (Fig. 1b).
During a pure protrusion or retraction event (we will use protrusion to represent both events below), the leading edge of the cell either extends to probe the environment or retracts back from a protrusion event if the anchorage to the ECM cannot be established while the nucleus remains immobile, i.e., NCD// ~ 0. Therefore, the CN correlation should be located within an angular zone around 90° in the polar coordinates of the CCD vs. NCD// plot. On the other hand, in a pure detachment event, the nucleus has roughly the same amount of coherent translocation as the trailing edge does; however, the relatively greater cell area in respect to the smaller nuclear area makes NCD// > CCD. Thus, a detachment event would be found in the angular zone below 45° of the plot. In addition, the leading edge protrusion and the trailing edge detachment can occur and be documented simultaneously within a monitored time interval. Hence, both detachment and protrusion events contribute to the CCD. When the protrusion event is significant to a certain level, the CCD value contributed from the protrusion event could make the overall CCD value greater than the NCD// value. This extra CCD would locate the corresponding CN correlation data point to the evasive migration zone, where it is in between the pure detachment zone and the pure protrusion zone. Moreover, a significantly negative NCD// (where the CCD and the coupled NCD form an obtuse angle) might imply a considerable turning event. The cell could markedly alter its CCD direction (more than 90°) from the previous one when a large size protrusion forms, whereas the nucleus retains its inertia in the previous direction. Hence, the NCD// could temporarily possess an opposite direction to the newly formed protrusion, resulting in a negative CN correlation.
These algorithms could provide an effective guideline to distinguish different types of subcellular activities of cell migration. Yet, this CN correlation concept does not come from a blind test. Each time, we need to monitor almost a complete cell locomotion event before we can determine the corresponding CCD and NCD for the analysis. However, each cell locomotion event takes different time to complete. Therefore, describing cell locomotion events using a fixed time lag cannot be achieved. Since a cell locomotion needs to be identified first before its duration can be determined, the CN correlation analysis can be validated only if it is independent from the durations of the locomotion events.
We therefore divide the entire cell motion into a series of motion fragments with fixed time intervals (e.g., 1 min). In this case, each locomotion during the whole cell motion period is also divided into a series of sub-locomotion events (subevents) with the same fixed time intervals. The subevents of a pure protrusion should possess the signature of the original protrusion, i.e., NCD// ~ 0. Similarly, a pure detachment should propagate its signature, NCD// > CCD, into its subevents. Shorter time intervals will also dissect an evasive migration event into a combination of subevents mixed with the signatures of pure protrusion and pure detachment events, and might be smaller evasive events. Finally, a large-angle turn can be well composed of evasive migration and large-angle protrusion, but must include some subevents with significantly negative NCD// as a signature. Thus, entire cell motions can be displayed as a collection of continuous, independent subevents from shorter, fixed time intervals. All the locomotion events could also be identified through the CN correlation analysis.
In essence, subcellular migratory activities might be simply composed of a series of subevents, classified into four independent modes in a CCD-NCD// coordinate system according to the corresponding CN correlation location — Region I (0° < θ ≤ 45°): Detachment Mode; Region II (45° < θ ≤ 75°): Mixture of Detachment and Protrusion Mode; Region III (75° < θ ≤ 105°): Protrusion/Retraction Mode; and Region IV (105° < θ ≤180°): Large-angle Side Protrusion Mode (Fig. 1b).
Barcodes demonstrate real time cell migration dynamics
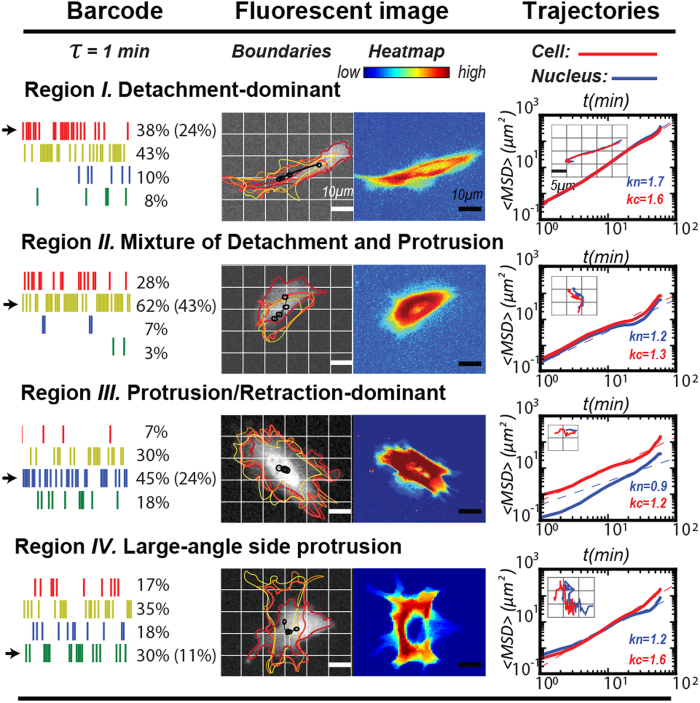
To test the hypothesis discussed above, we designed a barcode to display the migration information of a single cell (Fig. 1c). Movies of a single cell and its coupled nucleus were simultaneously acquired from RFP-introduced and Hoechst 33342-labeled NIH 3T3 fibroblast cultures at 1-min intervals over one hour, and were analyzed together to extract 60 CN correlation data points. Then, each CN correlation data point was assigned to a bar with a designated color based on the location of the point in the CCD vs. NCD// plot, where Regions I, II, III, and IV were specified as red, yellow, blue, and green, respectively. The bars were plotted horizontally by the time of occurrence and grouped vertically by region to assemble a barcode. Following, we analyzed 50 NIH 3T3 fibroblast movies, and obtained corresponding barcodes of each movie as a barcode collection (Supplementary Information 1). Using this collection, we calculated the overall occurrence rate of each region in all 50 samples, where occurrence percentage rates of Regions I, II, III, and IV were 24%, 42%, 24% and 11%, respectively. Consequently, we selected four individual barcodes in each separate region (Fig. 2, left), which possesses significantly higher percentile of CN correlation occurrence than the average occurrence rate of the corresponding region, and inspected their source movies.
Figure 2. Specific barcodes identify significant locomotion events during the cell migration.
In each row, a barcode, fluorescent image, and the trajectories of the cell and its nucleus are depicted in the left, middle, and right panels, respectively. Left: Each barcode, which displays the analysis results from its cell migration movie, was obtained from 1-min time intervals and possesses a dominant CCD-NCD// relationship in one of the four regions of the CN correlation. The percentage of the individual bars in the barcode representing each region is showed in the right column. The averaged occurrence of a region overall (50 barcodes) is shown inside the parenthesis. Middle: On the left, the cell boundaries from the 1st, 21st, 41st, and 61st fluorescent cell images are overlaid. Black markers represent the nuclear centroid positions. Red arrows indicate the migration directions of individual cells. On the right, heat map marks the distributions of cell territories in all 61 frames of the movie. The hot-to-cold color gradient illustrates the density of cell area occupancies. Right: A logarithm-logarithm plot of the overlapping mean squared displacement of the cells (red) and nuclei (blue) vs. time lag. Inset: one-hour cell and coupled nucleus trajectories of NIH 3T3 fibroblasts, colored in red and blue, respectively.
We analyzed these identified four cell movies (Supplementary Information 2: movie S1,2,3,4), which possess high occurrence rate of bars in a specific region, by tracing and overlaying the boundaries of the cells recorded at the 1st, 21st, 41st, and 61st (final) frames of each movie to monitor their migration statuses (Fig. 2, center left). To illustrate the locomotion patterns better, we also used the heat map to describe the variation of the cell boundaries from all 61 imaging frames of a cell movie (Fig. 2, center right). This analysis suggests that these four cells exhibited significantly different polarity properties by their protrusions/detachments. The cell with bars significantly increasing in Region I migrated persistently in one direction with protrusions concentrating on the leading edge and detachments concentrating on the trailing edge. The cell with bars having more occurrence rate in Region II displayed less directional persistence with wider protrusions in the leading edge. The cell with bars possessing more occurrence rate in Region III had protrusions probe in all directions. Finally, the cell with significant bars in Region IV showed protrusions and detachments mainly in two distinct directions, separated in a large angle. In general, the slope of the mean square displacement (MSD) indexes cell motility as a function of time lag30,31 (Fig. 2, right). The slope of the MSD revealed that the cell with bars significantly increasing in Region I underwent directional migration for both the cell and nucleus. In Region II and Region III, the cell displayed diffusive movement, but the nucleus exhibited sub-diffusive movement (stationary) in the Region III. In Region IV, the cell experienced a quick directional movement again, but the nucleus displayed normal diffusive motion. Hence, the application of the CN correlation analysis in a period of cell movie can identify the main locomotion events contributing to the cell migration period and, as a whole, signify the overall cell migration status displayed by the individual trajectories of these cells (Fig. 2, right, inset).
Evaluation of CN correlation analysis using a long-term single cell trajectory
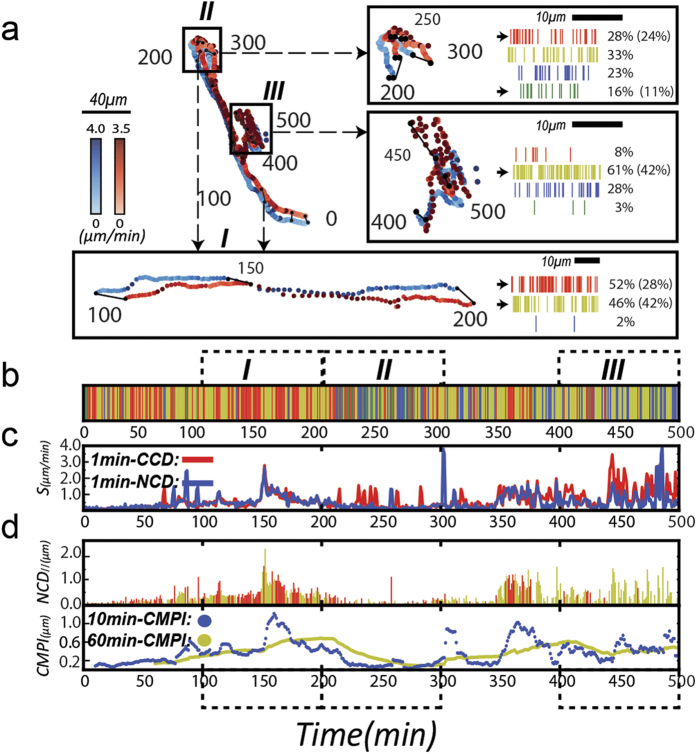
To further evaluate the CN correlation approach, the cell and coupled nuclear trajectories from a single NIH 3T3 fibroblast were displayed and analyzed at one-minute intervals for a 500-minute duration (Fig. 3a; also see Supplementary Information 2: movie S5). Effective cell migration events exhibited by the trajectories were generally consistent with the barcode. Again, the active migratory cell with straight trajectory had 52% of the bars in the barcode located in Region I, significantly greater than the average rate, 24% (Fig. 3a, Box I and the corresponding barcode). When a large-angled turning event occurred (200th–300th min in the trajectories), the corresponding barcode contained 16% of bars in Region IV, relatively higher than the average rate, 11%. The presence of a higher frequency of negative NCD// supported our previous prediction that CN correlation data in the Region IV link to large-angle side protrusions (Fig. 3a, Box II and the corresponding barcode). In fact, this would be the signature in the barcode of the cell turning event and the major difference to separate the cell turning event from the evasive cell migration event (Fig. 3a, Box III and corresponding barcode).
Figure 3. Long-term cell and nuclear trajectories and their correlations confirm the effectiveness of CN correlation approach in cell migration analysis.
(a) A 500-minute cell trajectory (red dots) and its coupled nuclear trajectory (blue dots) are displayed at one-min time intervals. Dots are displayed with color gradients to indicate the instantaneous speeds. Trajectories from 100th to 200th, 200th to 300th, and 400th to 500th minutes are separately re-drawn with the corresponding barcodes. Black lines illustrate the temporal relationship between the two trajectories by connecting the cell and the corresponding nuclear positions in 50-minute time points. (b) Trajectories of cells are presented as a region-mixed barcode. Bars are aligned by their time sequences and each bar indicates a predominant subcellular migratory activity: detachment-dominant (red), mixed detachment and protrusion (yellow), protrusion-dominant (blue) and large-angle side protrusion (green). (c) Instant nucleus (blue) and cell (red) migratory speeds are shown, calculated by one-min time sequences. (d) Upper Row: CN correlations that occurred in Region I (red) or II (yellow) are depicted, where the value of NCD// is plotted against time sequences. Bottom Row: Moving averages of CMPI are plotted against the time at 10-min (blue) and one-hour (yellow) intervals.
We further mapped the entire barcode with their instantaneous speeds. A 500-minute, region-mixed, barcode was drawn to illustrate the corresponding subcellular activities over the time course (Fig. 3b). Values of the instantaneous speed were also determined (Fig. 3c). These two charts revealed the correspondence of the detailed cell migration status. In the cases of evasive migration and turning, it can be observed that the cell momentarily moved at greater speed (red line) than the nucleus (blue line). This disparity of the speeds can correspond to bars with blue and green colors, which do not help expedite cell migration. In contrast, the comparable speeds occur when the bars are in red and yellow colors, where the cell and nucleus both moved together to promote cell migration.
These results are consistent with the prediction from the CN correlation analysis. In active cell motion, in which bars are distributed significantly in Region I and II, the cells and nuclei would have a strong, alternative corresponding locomotion. In cell turning events, the cells start with a large angle protrusion, followed with the nuclear direction change. In the evasive motion events, cells have less corresponding movements between the cells and nuclei with considerably more side protrusions, compared to the active motion.
Cell Migration potential index (CMPI) predicts mesenchymal-like cell motility
Based on the signatures of the subcellular migratory activities represented in the four regions of the CCD-NCD// coordinate system, we postulated that relative motility of mesenchymal-like cells could be gauged by a cell migration potential index (CMPI) according to the equation:
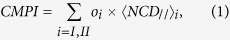 |
where oi denotes the occurrence of CN correlations over populations of the same cell type in a specific regioni, <NCD//>i represents the mean value of NCD// over populations of the same cell type in the specific region, and the subscript i represents either Region I or II in the CCD-NCD// coordinate system. According to this definition, a greater CMPI value of a cell should indicate a greater motility.
The CN correlations identify effective cell movements by extracting significant nuclear translocations in the moving direction of the cells, which are located in the Region I and II of the coordinate system. In contrast, nuclear translocations in Region III or IV do not directly contribute to cell motility and can be ignored. The CMPI was designed to capture the effective nuclear movement events, represented by the NCD// shown in Region I and II (red and yellow, respectively) over the time course (Fig. 3d, top row). Once those NCD// values were accumulated as an index for cell motility, the short-term running CMPI (e.g., 10 minutes here) can reveal the migration details of the cells, whereas the long-term running CMPI (e.g., 60 minutes here) approach a stable value that might represent the overall cell motility (Fig. 3d, bottom row).
Taken together, CN correlation analysis might assess cell motility in real time and, as the analysis shows, each CN correlation datum can represent the corresponding subcellular locomotion mode.
Evaluation of CMPI using the Persistent Random Walk (PRW) model
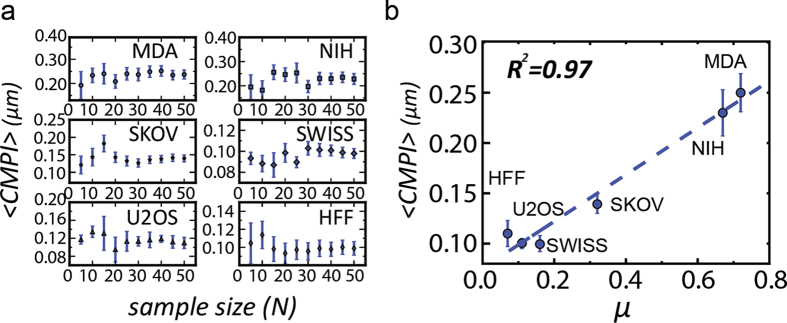
We hypothesized that the averaged CMPI value (expressed as <CMPI>) from multiple cells of a cell type might be used to represent the motility of the cell type. To test this hypothesis, we first evaluated the precision of <CMPI> against the cell sample size. The cell types studied included 3 cancer cell lines: MDA-MB-231, SKOV-3, and U-2 OS cells, and 3 fibroblast lines: NIH 3T3, Swiss 3T3, and human foreskin fibroblasts. <CMPI> of each cell type was calculated from different cell sample sizes. At each iteration, samples of each cell type were independently and randomly chosen from a pool of 50 samples and cell sample size was increased by a step of 5. At small sample sizes (1-min time intervals and one-hour monitoring time), the <CMPI> of all six cell types fluctuated. However, when the sample size exceeded 20, the <CMPI> approached a steady state, in which the standard error of the mean diminished to the confidence limit (less than 10% of the mean value) (Fig. 4a). These results indicate that a stable <CMPI>, i.e., exhibiting a good convergent tendency, can be obtained from a relatively small cell sample size (~20).
Figure 4. A sample size of 20 one-hour cell migration movies is sufficient to derive precise and accurate CMPI for cell motility description.
(a) Means and variances of CMPI are plotted against sample size for 6 different types of cells. Error bars represent standard error of the mean. (b) Correlation between CMPIs and diffusion coefficients for 6 different cell types. A blue dot represents a cell type, and the dashed line is the fitting curve. Abbreviations for cell types: MDA-MB-231 (MDA), NIH 3T3 (NIH), SKOV-3 (SKOV), OVCAR-3 (OVCAR), U-2 OS (U2OS), SWISS 3T3 (SWISS) and Human Foreskin Fibroblasts (HFF).
The <CMPI> and standard errors of each cell type were calculated from data extracted from 50 one-hour cells and coupled nuclei movies, acquired at one-min time intervals, to verify the accuracy of CMPI in describing the trends of cell motility among different cells (Table 1). These data were compared to their corresponding diffusion coefficients, μ, obtained from the Persistent Random Walk (PRW) model17,32,33. The diffusion coefficient of each cell type was extracted from ~15 nuclear trajectories, tracked at 1-min time intervals for 10 hours. The fitting between the diffusion coefficient and CMPI produced an R2-value of 0.97 (Fig. 4b). Hence, this result demonstrates that CMPI provides an excellent estimate of the motility of mesenchymal-like cell types, and indicates that the motility of single mesenchymal-like cells can be efficiently described at short time intervals under the CN correlation approach using the cell migration potential index, CMPI.
Table 1. Cell migration potential index (CMPI) from 6 different cell types.
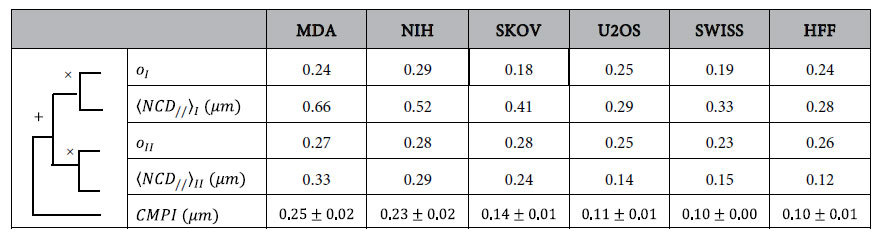
CMPI values were calculated according to the equation: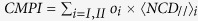 . Input data were acquired at one-min time intervals from one-hour movies. N = 50 cells.
. Input data were acquired at one-min time intervals from one-hour movies. N = 50 cells.
Values are reported as mean ± SE (standard error) from 50 cells.
MDA, NIH, SKOV, OVCAR, U2OS, SWISS, HFF is the abbreviation of cell lines MDA-MB-231, NIH 3T3, SKOV-3, OVCAR-3, U-2 OS, SWISS 3T3 and Human Foreskin Fibroblast, respectively.
The impact of positioning error in the subcellular activity classification
When acquired from shorter time intervals, the magnitudes of the CCDs and NCDs inevitably become smaller. Hence, the imaging acquisition errors will have more significant impacts to the data. To evaluate this impact, a fixed-cell was subjected to the CN correlation analysis to obtain the positioning error profile in the CCD-NCD// coordinate system. Since the probed cell was fixed, the non-zero distribution of its CN correlation data in the CCD vs. NCD// plot was merely contributed from the acquisition errors, which formed a special region in the CCD-NCD// coordinate system that could be treated as a separate distribution, called Region V: Stationary Mode. The results confirmed that the positioning error cannot be ignored (Fig. 5a). However, it cannot be an implication that every CN correlation datum located within the zone is in stationary mode since the positioning error could also make a non-zero CN correlation datum shift and look “stationary”.
Figure 5. CMPI estimates are independent of positioning errors.
(a) CCD and coupled NCD// of 50 single live NIH cells (red dot) and fixed NIH cells (blue dots) in the CCD vs. NCD// plot. (b) Left: Original barcode of an individual NIH cell tracked at 1-min lag time for 60 min. Right: Error-filtered barcode of the same tracking data. (c) Linear relationship of diffusion coefficient and error-filtered CMPI.
Hence, we used the Monte Carlo method to create a probability density function by the distribution of the fixed-cell data, denoted as p(NCD//,CCD), to evaluate whether a CN correlation datum generated from a live-cell belongs to the stationary mode. Afterwards, we randomly generated decimals in range [0, 1] for each live-cell datum (denoted as subscript i). If this random value is greater than the noise probability, pi(NCD//,CCD), the datum is treated as an effective motion mode. If the random value exhibits a converse property, it will be considered as an ineffective motion mode, as shown below.
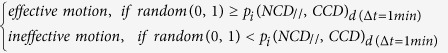 |
Through this approach, whether a datum belongs to the stationary mode can be identified.
We further evaluate whether the previous CMPI estimate might be biased without considering the CN correlation data in the stationary mode. We defined a new fifth row in the barcode as Region V, in which the bars are labeled in black. We then re-probed cells, and shifted those ineffective subcellular modes from other regions into Region V (Fig. 5b). After using the five-region barcode for CN correlation analysis, we continued comparing the new CMPI values and the diffusion coefficients derived from the PRW model in six mesenchymal-like cell lines. The linear fitting has an R2 value = 0.97 (Fig. 5c, detailed data see Supplementary Information 3), which is comparable to that from the four-region barcode. Hence, this practice supported that the absorption of those insignificant stationary modes into the four-region barcode will not impact the CMPI estimate to describe the motility trends among different mesenchymal-like cell lines. In addition, unless a cell stays in the stationary mode for a prolonged time (e.g., more than five minutes), we may not consider the cell as being in the stationary state. When cells probed in such a short time lags (e.g., 1-min), a normal locomotion event could have a certain discrete period (1–3 minutes) showing in the stationary mode.
Robustness of CMPI analysis
To obtain correct CMPI, we applied fixed particles to the glass-bottom dishes as reference points among image frames to minimize positioning errors generated during routine computer-controlled data acquisition using a particle tracking technique34. We also developed a Gaussian filter algorithm to identify the cell boundary with a level of accuracy that cannot be achieved using the normal segmentation process35. Consequently, the errors generated during image acquisition and analysis could be greatly reduced. Nonetheless, intrinsic errors are still present during image acquisition. These intrinsic errors may obscure the true displacement of cells or nuclei if the observation time intervals are too short. However, if the observation period is too long, individual subcellular activities would be overlooked, again generating inaccurate estimates of CMPI. Hence, an optimal temporal setting is needed to be set for the documentation of individual subcellular activities. Therefore, a series of robustness tests were applied to determine the appropriate time intervals for image documentation and to examine the effects of intrinsic errors (noise) on CMPI.
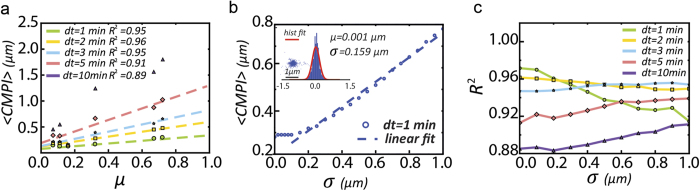
To determine the optimal time intervals at which to record the CMPI, the CMPI of the 6 mesenchymal-like cell types were calculated at different time intervals, τ = 1, 2, 3, 5, and 10 min, from 50 cells during a one-hour monitoring time. The resultant CMPI were linearly fitted to the corresponding PRW-derived diffusion coefficients (τ = 1 min) to determine the time interval at which the derived CMPI most closely correlated with the motility trends of different cells acquired via the PRW model. The results showed that the most consistent trends between the CMPI and their corresponding diffusion coefficients (with an R2 value ≥ 0.95) occurred at time intervals of 1 to 3 minutes (Fig. 6a).
Figure 6. One to three-minute time intervals can describe the robust CMPI utilizing various image acquisition systems.
(a) CMPI of 6 different cell types calculated at 1, 2, 3, 5, or 10 min time intervals are compared against the corresponding diffusion coefficients, μ. (b) Random Gaussian noise-implemented CMPI values are plotted against the standard deviation of the Gaussian noise (ranging from 0–1.0 μm). The initial data were obtained from NIH 3T3 fibroblasts at 1-min time intervals. Inset: The distribution of system noise was determined from the centroid positions of a fixed cell. (c) The fitting results (R2) between noise-implemented CMPI and corresponding diffusion coefficients, μ, of 6 cell types were plotted against the standard deviation of introduced noise (ranging from 0–1.0 μm). CMPI were calculated using data extracted at 1-, 2-, 3-, 5-, and 10-min intervals from 1-hour movies of the 6 different cell types.
We then evaluated the impact of the intrinsic system noise on the estimation of CMPI. To do so, we first determined the contribution of intrinsic errors (i.e., the uncontrollable errors) generated in our microscopy system. Since the correctable errors generated from the image acquisition and analysis process had been minimized by fixed particles, we attributed the remaining factors that resulted in inconsistent centroid positions of a fixed cell to intrinsic system errors. We repeatedly measured the centroid positions of fixed cells to obtain the distribution and variance of the centroid positions, which displayed as a Gaussian distribution in both x- and y-coordinates with the mean at the center and the standard deviation (σ) = 0.17 μm and 0.16 μm, respectively (Fig. 6b, inset). Here, the standard deviation of the distributions (~0.2 μm) can be treated as the intrinsic errors of our microscopic systems.
We assessed the impact of the intrinsic system noise on the estimation of CMPI. To approach this, a randomly generated Gaussian noise (with the mean = 0 and standard deviation = σ) was applied to each of the experimental CCD and NCD data points of NIH 3T3 fibroblasts to distort the displacement measurements (for this analysis, the experimental data were assumed not to possess any errors).
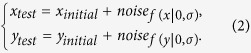 |
After this procedure, an error-implemented CMPI was calculated. The resulting <CMPI> values, obtained from 1000 repeats of noise-implemented CMPI determinations at a given σ (ranging from 0.02 to 1 μm), showed that the system noise had no significant impact on the <CMPI> values when σ was less than 0.15 μm but exhibited a linear correlation as σ increased beyond 0.15 μm (Fig. 6b). This result suggests that <CMPI> determinations are influenced by the intrinsic system errors in a linear manner when the image acquisition system possesses an intrinsic system error greater than 0.15 μm.
Since the CMPI provides relative motility among different cell types, the relevant issue is whether different acquisition systems (with different intrinsic system errors) will generate very different CMPI results that would affect the comparison of cell motility among cells. Hence, we further assessed the competence for using CMPI to judge the relative motility among different cell types in the presence of different sizes of system errors. The noise-implemented <CMPI> of the 6 cell types were then calculated at different σ values (ranging from 0 to 1 μm) at various time intervals (1, 2, 3, 5, and 10 min) and linearly fitted to the corresponding diffusion coefficients. The results indicate that the CMPI calculated from τ = 1–3 min accurately described motility trends (R2 ~ 0.96) if the system noise (σ) was <0.3 μm. CMPI calculated from τ = 2–3 min can consistently describe motility trends when the noise was between 0.3 and 0.8 μm (Fig. 6c).
The study presented here demonstrates that the CN correlation approach can be used to accurately and reliably estimate cell motility. CMPI is a robust index of cell motility for various cell types, and is resistant to intrinsic noise carried by a normal microscopic system. Hence, CMPI is a biologically relevant index for mesenchymal-like cells, linking cell motility to corresponding subcellular activities.
Discussion
In this study, we introduce a novel CN correlation approach to analyzing cell migration, in which the motion of the cell relative to the motion of the nucleus is utilized to classify and weight the stages of cell migration into four momentary subcellular events: (1) detachment dominant, (2) mixed detachment and protrusion, (3) protrusion dominant, and (4) large-angle side protrusion. We adopt a barcode system to assist in the visualization of dynamic processes and their temporal occurrence in single cells. We also define a quantitative cell migration potential index (CMPI) to characterize these subcellular activities. Using CMPI, cell migration can be deciphered in detail, and cell motility can be delineated according to discrete biological events. This approach provides an avenue to connect cell migration to its underlying subcellular activities, explains the effects of associated molecular events, and further delivers a powerful tool to associate individual molecular mechanisms with their ensemble cellular activities.
Reliable cellular measurements are essential to the CN correlation approach. Identification of correct cell boundaries, including thin lamellipodia, to determine centroid information during imaging analysis is a prerequisite procedure. It is vital to select appropriate time intervals that not only support highly resolved individual subcellular documentation, but also distinguish cell and nuclear displacements from background noise. In previous studies34,35, we provided detailed imaging processing guidelines to ensure successful CN correlation analysis. Since we adopt short time intervals in this study, the accuracy of boundary information is critical. The short time intervals further fragment the subcellular locomotion events into smaller subevents, in which the displacements between the time frame could be largely affected by the errors generated through the imaging acquisition and boundary determination. Hence, a real stationary mode cannot be easily separated from small motion. We therefore provide a Monte Carlo approach and use a five-region barcode to separate the stationary modes from small motions to determine the impact of the stationary modes. Since these small motions are more likely to appear in Region II and Region III, identification of active motions from subevents would not be affected much; hence, we mainly adopt the four-region barcode in this study to build up the migration capability index. This choice has been demonstrated to preserve the integrity of the cell migration states and retain cell motility trends accurately among different cell lines. In case the stationary state needs to be specifically identified for a certain purpose, Region V can be applied to the barcode to isolate continuous stationary modes.
In this work, we develop CMPI and demonstrate the reliability and adaptability of CMPI. The cells often move in the 3-dimensional (3-D) environment under physiological conditions, in which the physiological morphology and stiffness of the extracellular matrix vary broadly to affect cell motility. Hence, it would be difficult to claim a reliable quantitative method to link subcellular activities and assess cell motility across various environmental conditions. On the other hand, the CN correlation approach introduced here can smoothly deliver a precise, quantitative description to link subcellular activities (therefore underlying signaling pathways) to cell motility for fundamental biomedical study and the pharmaceutical industry.
Currently, the wound-healing assay and the PRW model are the two main methods used for comparison of migration potentials among related cell types. The wound-healing assay is a straightforward method to assess the capacity of cells grown to repopulate (heal) a mechanically introduced cell-free zone through collective cell migration and proliferation. In the wound-healing assay, cell migration (and/or proliferation) is quantified as the void area occupied by cells. The PRW model, on the other hand, treats individual cells as particles, and utilizes their mean squared displacements (MSDs) to characterize cell motility. Through combining these two cell migration assessments with molecular manipulation, our understanding of cell migration at both cellular and molecular levels has greatly advanced. However, in both of these systems, biophysical information that details the subcellular events, which guide distinct cell migration patterns and account for en route cell motility changes, are not assessed directly. The CN correlation approach can comprehensively incorporate the subcellular events into cell migration analysis.
In this study, we use CMPI to analyze and compare the trends of six cell types. We show that the motility trends of the six cell types overall determined from CMPI and PWR are highly consistent, with R2 > 0.95. Since the PWR model is derived from a mathematic approach, several specific assumptions regarding cell migration have been proposed in this model. These assumptions include instantaneous speed being time-independent, Gaussian distribution of velocities, exponential decay for the autocorrelation function, and isotropic cell movement. However, these assumptions cannot be always satisfied for different cell types due to heterogeneous cell behaviors. Therefore, the mathematical procedures need to be constantly modified to match varying cell migratory features36,37. In contrast, the CMPI approach requires no prior assumptions, but is directly rooted in the physiological behavior of the cells with straightforward mechanical analysis.
The CN correlation and PRW model both utilize trajectory information to realize mesenchymal-like cell migration. The PRW model utilizes speed (S2) and persistence time (P) as two main parameters to determine cell migration capacity. For accurate migration analyses, a long tracking time (~10 hrs) is required. The occurrence of photo-bleaching from extensive light exposure during the tracking period can interfere with the analysis. In contrast, the CN correlation approach analyzes migratory behavior of individual cells over a relatively short monitoring time (e.g., 1 hour), and requires small sample sizes (approximately 20 cells). Therefore, the assay conditions are more favorable toward reducing photobleaching effects and experimental time.
In addition, CMPI is not limited to describing the motility of a mesenchymal cell type; it should also be used to describe the dynamic processes of any individual cell in real time. Every protrusion or contraction event can be separately and sequentially recorded in a barcode. When a barcode is applied to describe the migratory process of a single cell, the barcode and CMPI can facilitate the visualization of the individual cell migration process in a digitized computer format. However, the CN correlation approach can be exploited. For example, in analyzing kinetic processes during drug therapy, this approach can provide information regarding the impact of a drug on subcellular and cellular processes during cell motion as a cell dynamic assessment method for applications such as clinical trials.
Based on the presenting capacity of the CN correlation approach to mapping different subcellular activities in a cell migration event, we further provide the autocorrelation information among the CN correlation data. CN correlation contains two types of information: angular values (to identify what type of locomotion) and NCD// (to indicate nuclear speed) through the CCD-NCD// coordinate system. We conduct the autocorrelation among these two types of information by three time intervals (1–3 min) and in the four different significant locomotion cases showed in Fig. 2. The results of the angular autocorrelations indicate whether a temporal pattern exists within a cell locomotion event while the NCD// autocorrelations evaluate the consistence of the nuclear speed or a specific repeated pattern is present. The results (Supplementary Information 4) confirmed that clear NCD// autocorrelations exist in all four cell locomotion cases, which verifies the availability of CN correlation analysis. In opposite, the angular autocorrelations are fuzzy, mainly owing to its nonlinear nature in describing the subevents of the locomotion. Although these two types of autocorrelations are seemingly disjoint to each other, they should have a certain type of connection through the NCD// values (i.e., the NCD// should possess inertial to prevent an abrupt change in cell locomotion). Thus, we will study the connection between these two types of autocorrelations to recognize migration patterns in the future work. In addition, 1–3 min time interval analysis results also have a consistent correlation trends, supporting the validity of CN correlation data at different time analysis scales.
In conclusion, wound healing scratch assays may be better for high throughput, whereas CMPI would be better for studying cancer-related EMT gene expression, etc. wherein the molecular mechanisms are important. The results of this study provide an effective and efficient means to analyze mesenchymal-like cell migration.
Methods
Cell cultures, plasmid transfection, and nuclear stain
MDA-MB-231, NIH 3T3, and Swiss 3T3 cell lines and human foreskin fibroblasts (all purchased from ATCC, Manassas, VA) were cultured in DMEM containing 10% fetal bovine serum (FBS) and 1% L-glutamine (Mediatech). U-2 OS cells (ATCC) were cultured in McCoy’s 5A medium with 10% FBS and 1% L-glutamine (Mediatech). Cultured cells were maintained in a humidified incubator at 37 °C and 10% CO2. pRFP-R-CS plasmids (Origene, Rockville, MD), containing red fluorescence protein (RFP) gene, were introduced into cells using Lipofectamine 2000 (Invitrogen, Carlsbad, CA) following the standard protocol. Subsequently, the cells were seeded on fibronectin (BD Biosciences, San Jose, CA)-coated glass bottom dishes for 24 hours prior to image acquisition. Live-cell nuclear stain, Hoechst 33342 (Sigma-Aldrich, St. Louis, MO), was added to the medium 10 minutes prior to image acquisition.
Microscopy
A Nikon TE-2000 microscope (Nikon, Melville, NY), equipped with an X-Cite 120PC fluorescent light source (EXFO, Ontario, Canada), a Cascade: 1 K charge coupled digital camera (Roper Scientific, Tucson, AZ), and an on-stage incubator with CO2 supplementary system (In Vivo Scientific, St. Louis, MO), was used to acquire images of cell and nucleus motion. The image acquisition environment was kept at 10% CO2 and 37 °C during experiments.
The fluorescent images of cells and the concomitant nuclei were sequentially acquired using a 20× objective lens (Nikon) at a frequency of one-minute per acquisition cycle. For cell migration potential index (CMPI) determination, movements of cell and nucleus were acquired over one hour in general case. An acquisition over 500 minutes was also applied for long-term analysis. For persistence time calculation, the nuclear trajectories were acquired over ten hours. Microscopy parameters were 100 ms exposure time, 3 × 3 bin size, and 25% power of light source.
Single cell trajectory analysis
Cell and nucleus centroids were determined from the sequentially acquired images based on segmentation performed on each image frame. Subtle changes in cell morphology were faithfully captured using our previously developed spatial filtering scheme35. The measured centroid can accurately reflect those changes, enhancing the spatial resolution of cell and nucleus trajectories. A trajectory was determined by linking the geometric centers of the tracked object through the time sequence. The long-time (10-hour) nuclear trajectories served as the source information to calculate persistence time using a custom-made program in MATLAB (Mathworks, Natick, MA). The persistence time of each cell type was determined by fitting the mean square displacement, 〈r2〉, with root mean square speed, S, using the persistent random walk equation: 〈r2〉 = 2S2P[t − P(1 − e−t/P)], where P and t are the persistence time and time interval, respectively38,39. We adopted Levenberg-Marquardt algorithm to perform the least square fitting since it gave the best fitting results for our cases. The diffusion coefficient, μ = 〈S2〉P/n, where n (=2 here) is the dimension of extracellular space, was derived from P and 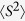 17.
17.
Additional Information
How to cite this article: Lan, T. et al. Displacement correlations between a single mesenchymal-like cell and its nucleus effectively link subcellular activities and motility in cell migration analysis. Sci. Rep. 6, 34047; doi: 10.1038/srep34047 (2016).
Supplementary Material
Acknowledgments
This study was supported in part by grants from NIH/NCI U54CA143868. Stephen Hugo Arce was partially supported by a McKnight Doctoral Fellowship. The authors thank Dr. Shen-hsiu Hung for his suggestion and assistant to collect the data.
Footnotes
Author Contributions T.L. and Y.T. designed experiments, analyze the data and wrote the manuscript. T.L., K.C. and T.R. conducted experiments and collected data. T.L., Y.T. and S.H.A. discussed the results.
References
- Richardson B. E. & Lehmann R. Mechanisms guiding primordial germ cell migration: strategies from different organisms. Nat Rev Mol Cell Biol. 11, 37–49, doi: 10.1038/nrm2815 (2010). [DOI] [PMC free article] [PubMed] [Google Scholar]
- George E. L., Georges-Labouesse E. N., Patel-King R. S., Rayburn H. & Hynes R. O. Defects in mesoderm, neural tube and vascular development in mouse embryos lacking fibronectin. Development 119, 1079–1091 (1993). [DOI] [PubMed] [Google Scholar]
- Grose R. et al. A role for endogenous glucocorticoids in wound repair. EMBO Rep. 3, 575–582, doi: 10.1093/embo-reports/kvf119 (2002). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Martin P. & Parkhurst S. M. Parallels between tissue repair and embryo morphogenesis. Development 131, 3021–3034, doi: 10.1242/dev.01253 (2004). [DOI] [PubMed] [Google Scholar]
- Werner S. & Grose R. Regulation of wound healing by growth factors and cytokines. Physiol Rev. 83, 835–870, doi: 10.1152/physrev.00031.2002 (2003). [DOI] [PubMed] [Google Scholar]
- Friedl P. & Wolf K. Tumour-cell invasion and migration: diversity and escape mechanisms. Nat Rev Cancer. 3, 362–374, doi: 10.1038/nrc1075 (2003). [DOI] [PubMed] [Google Scholar]
- Gupta G. P. & Massagué J. Cancer metastasis: building a framework. Cell 127, 679–695, doi: 10.1016/j.cell.2006.11.001 (2006). [DOI] [PubMed] [Google Scholar]
- Bacac M. & Stamenkovic I. Metastatic cancer cell. Annual Review of Pathology-Mechanisms of Disease 3, 221–247, doi: 10.1146/annurev.pathmechdis.3.121806.151523|10.1146/annurev.pathol.3.121306.151523 (2008). [DOI] [PubMed] [Google Scholar]
- Chambers A. F., Groom A. C. & MacDonald I. C. Dissemination and growth of cancer cells in metastatic sites. Nat Rev Cancer 2, 563–572, doi: 10.1038/nrc865 (2002). [DOI] [PubMed] [Google Scholar]
- Kwang S. J. et al. Msx2 is an immediate downstream effector of Pax3 in the development of the murine cardiac neural crest. Development 129, 527–538 (2002). [DOI] [PubMed] [Google Scholar]
- Siegel R., Naishadham D. & Jemal A. Cancer statistics, 2013. CA Cancer J Clin. 63, 11–30, doi: 10.3322/caac.21166 (2013). [DOI] [PubMed] [Google Scholar]
- Todaro G. J., Lazar G. K. & Green H. The initiation of cell division in a contact-inhibited mammalian cell line. J Cell Physiol 66, 325–333 (1965). [DOI] [PubMed] [Google Scholar]
- Lipton A., Klinger I., Paul D. & Holley R. W. Migration of mouse 3T3 fibroblasts in response to a serum factor. Proc Natl Acad Sci USA 68, 2799–2801 (1971). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee H., Goetzl E. J. & An S. Lysophosphatidic acid and sphingosine 1-phosphate stimulate endothelial cell wound healing. Am J Physiol Cell Physiol. 278, C612–618 (2000). [DOI] [PubMed] [Google Scholar]
- Yin J. et al. The Effect of Z-Ligustilide on the Mobility of Human Glioblastoma T98G Cells. PLoS One. 8, e66598, doi: 10.1371/journal.pone.0066598 (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Codling E. A., Plank M. J. & Benhamou S. Random walk models in biology. J R Soc Interface 5, 813–834, doi: 10.1098/rsif.2008.0014 (2008). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stokes C. L., Lauffenburger D. A. & Williams S. K. Migration of individual microvessel endothelial cells: stochastic model and parameter measurement. J Cell Sci. 99 (Pt 2), 419–430 (1991). [DOI] [PubMed] [Google Scholar]
- Burridge K. & Wennerberg K. Rho and Rac take center stage. Cell 116, 167–179 (2004). [DOI] [PubMed] [Google Scholar]
- Lauffenburger D. A. & Horwitz A. F. Cell migration: a physically integrated molecular process. Cell 84, 359–369 (1996). [DOI] [PubMed] [Google Scholar]
- Sahai E. & Marshall C. J. Differing modes of tumour cell invasion have distinct requirements for Rho/ROCK signalling and extracellular proteolysis. Nat Cell Biol 5, 711–719, doi: 10.1038/ncb1019 (2003). [DOI] [PubMed] [Google Scholar]
- Condeelis J. Life at the leading edge: the formation of cell protrusions. Annu Rev Cell Biol 9, 411–444, doi: 10.1146/annurev.cb.09.110193.002211 (1993). [DOI] [PubMed] [Google Scholar]
- Chen W. T. Mechanism of retraction of the trailing edge during fibroblast movement. J Cell Biol. 90, 187–200 (1981). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mullins R. D., Heuser J. A. & Pollard T. D. The interaction of Arp2/3 complex with actin: nucleation, high affinity pointed end capping, and formation of branching networks of filaments. Proc Natl Acad Sci USA 95, 6181–6186 (1998). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ren X. D., Kiosses W. B. & Schwartz M. A. Regulation of the small GTP-binding protein Rho by cell adhesion and the cytoskeleton. EMBO J. 18, 578–585, doi: 10.1093/emboj/18.3.578 (1999). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kaverina I., Krylyshkina O. & Small J. V. Microtubule targeting of substrate contacts promotes their relaxation and dissociation. J Cell Biol. 146, 1033–1044 (1999). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ballestrem C., Hinz B., Imhof B. A. & Wehrle-Haller B. Marching at the front and dragging behind: differential alphaVbeta3-integrin turnover regulates focal adhesion behavior. J Cell Biol. 155, 1319–1332, doi: 10.1083/jcb.200107107 (2001). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Machacek M. et al. Coordination of Rho GTPase activities during cell protrusion. Nature 461, 99–103, doi: 10.1038/nature08242 (2009). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Razafsky D. & Hodzic D. Bringing KASH under the SUN: the many faces of nucleo-cytoskeletal connections. J Cell Biol. 186, 461–472, doi: 10.1083/jcb.200906068 (2009). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee J. S., Chang M. I., Tseng Y. & Wirtz D. Cdc42 mediates nucleus movement and MTOC polarization in Swiss 3T3 fibroblasts under mechanical shear stress. Mol Biol Cell 16, 871–880, doi: 10.1091/mbc.E03-12-0910 (2005). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Qian H., Sheetz M. P. & Elson E. L. Single particle tracking. Analysis of diffusion and flow in two-dimensional systems. Biophys J. 60, 910–921, doi: 10.1016/S0006-3495(91)82125-7 (1991). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Saxton M. J. & Jacobson K. Single-particle tracking: applications to membrane dynamics. Annu Rev Biophys Biomol Struct. 26, 373–399, doi: 10.1146/annurev.biophys.26.1.373 (1997). [DOI] [PubMed] [Google Scholar]
- Gail M. H. & Boone C. W. The locomotion of mouse fibroblasts in tissue culture. Biophys J 10, 980–993, doi: 10.1016/S0006-3495(70)86347-0 (1970). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dickinson R. B., McCarthy J. B. & Tranquillo R. T. Quantitative characterization of cell invasion in vitro: formulation and validation of a mathematical model of the collagen gel invasion assay. Ann Biomed Eng. 21, 679–697 (1993). [DOI] [PubMed] [Google Scholar]
- Wu P. H., Arce S. H., Burney P. R. & Tseng Y. A novel approach to high accuracy of video-based microrheology. Biophys J. 96, 5103–5111, doi: 10.1016/j.bpj.2009.03.029 (2009). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Arce S. H., Wu P. H. & Tseng Y. Fast and accurate automated cell boundary determination for fluorescence microscopy. Sci Rep. 3, 2266, doi: 10.1038/srep02266 (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wu P. H., Giri A., Sun S. X. & Wirtz D. Three-dimensional cell migration does not follow a random walk. Proc Natl Acad Sci USA 111, 3949–3954, doi: 10.1073/pnas.1318967111 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kipper M. J., Kleinman H. K. & Wang F. W. New method for modeling connective-tissue cell migration: improved accuracy on motility parameters. Biophys J. 93, 1797–1808, doi: 10.1529/biophysj.106.096800 (2007). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dunn G. A. Characterising a kinesis response: time averaged measures of cell speed and directional persistence. Agents Actions Suppl. 12, 14–33 (1983). [DOI] [PubMed] [Google Scholar]
- Othmer H. G., Dunbar S. R. & Alt W. Models of dispersal in biological systems. J Math Biol. 26, 263–298 (1988). [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.