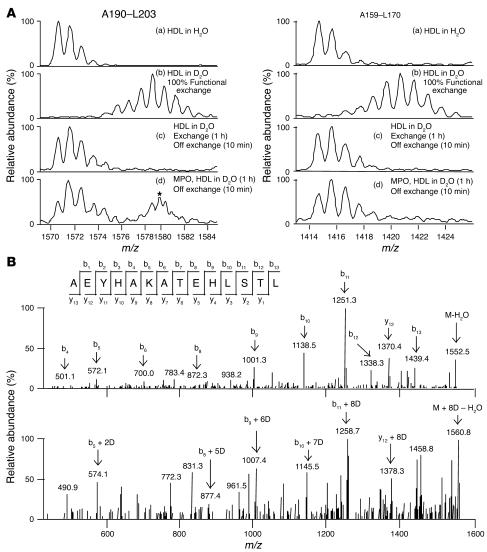
Figure 5.
Demonstration of an interaction between MPO and apoA-I using hydrogen/deuterium exchange tandem MS. Exchangeable protons on HDL and MPO were each deuterium-labeled by mixing in D2O containing ND4OAc, pD 7.0, at room temperature for 1 hour. The deuterated HDL was combined with either deuterated MPO or additional deuterium buffer and incubated for 1 hour at room temperature to allow binding. Samples were diluted 25-fold into NH4OAc, pH 7.0, for 10 minutes for back (off) exchange before quenching by rapid cooling and addition of TFA to pH 2. Proteins were digested with immobilized pepsin, and then samples were immediately injected for analysis as described in Methods. The different spectra shown correspond to various stages in the hydrogen/deuterium exchange experiment. (A) ApoA-I isotopic clusters shown are for either the MPO-binding peptide A190–L203 (left) or a negative control peptide, L159–L170 (right). For each, spectra a and b contain peptide isotopic clusters before and after deuterium labeling, respectively. Spectra c and d contain deuterium-labeled peptide cluster after back (off) exchange with hydrogen in the absence and presence of MPO binding, respectively. The peptide isotopic cluster indicated by the asterisk represents a non–back-exchanged component of the A190–L203 isotope cluster due to inaccessibility of this region of apoA-I to solvent in the presence of MPO. Results shown are representative of 4 independent experiments. (B) Sequence confirmation of the identified peptic peptides was achieved by tandem MS. The collision-induced dissociation spectra and fragmentation analysis of the unlabeled and deuterium-labeled peptic peptide A190–L203 are illustrated.