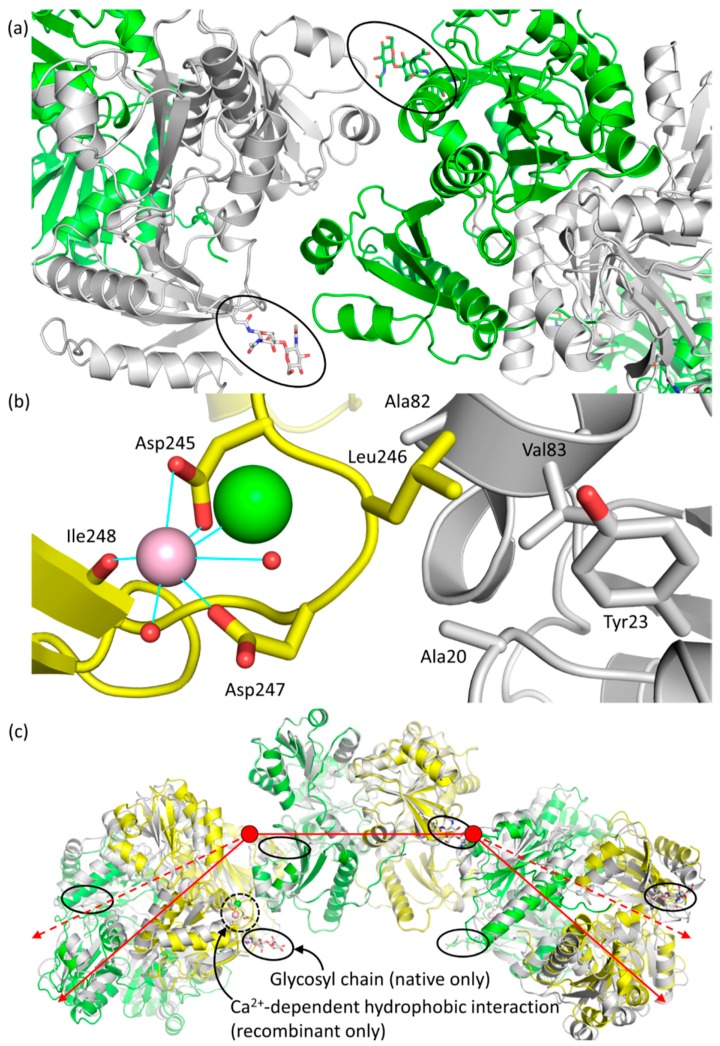
Figure 7.
(a) An overhead view of a back-to-back interface showing the positioning of the glycosyl chains and the apparent steric constraints that they place on the polymer; (b) Close up of the Ca2+-dependent hydrophobic interaction seen in recombinant bovine Casq1. Relevant residues are labeled. The pink spheres represent Ca2+ ions and the green sphere represents a chloride ion, and the two small red spheres represent water molecules. Solid blue lines represent coordination bonds observed in the electron density map; (c) Least squares superposition of Cα atom in monomers A and A′ (central dimer) between their native hexameric form (from left to right, green, gray, green, gray, green and gray) and recombinant hexameric form (from left to right, gray, yellow, gray, yellow, gray and yellow). Glycosyl chains are circled with a black line, and the Ca2+-dependent hydrophobic interaction, only seen in high-Ca2+ recombinant, is circled with a black dotted line. The red lines are two-dimensional vector representations of the three dimers that compose the hexamer, with the dashed red lines indicating the deviation of recombinant Casq1 from native. In three-dimensional space and from a side-on perspective, each dimer of native Casq1 is rotated 18° with respect to the point at which two dimeric vectors meet (red circle) within their back-to-back interface, whereas recombinant Casq1 is rotated by only one.