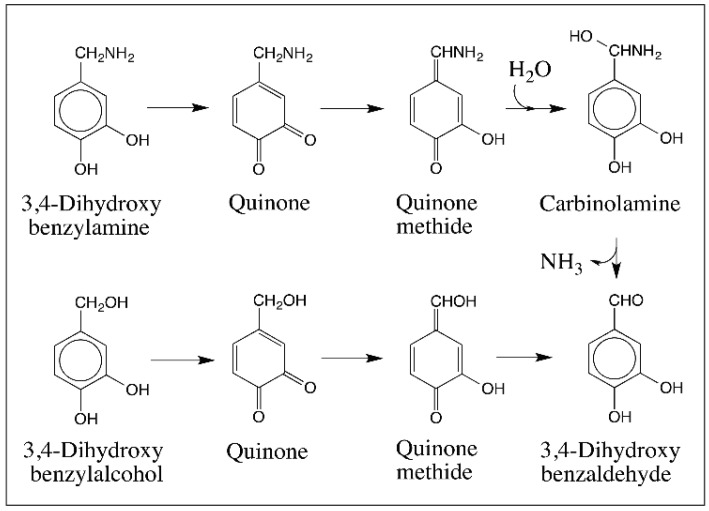
Figure 12.
Oxidation chemistry of 3,4-dihydroxybenzylamine and 3,4-dihydroxybenzyl alcohol. 3,4-Dihydroxybenzylamine is easily oxidized to its quinone, which undergoes rapid nonenzymatic isomerization to quinone methide. Quinone methide reacts with water to form carbinolamine and then looses ammonia generating 3,4-dihydroxybenzaldehyde as the final product. A similar conversion of 3,4-dihydroxybenzyl alcohol to 3,4-dihydroxybenzaldehyde also occurs through a quinone methide intermediate.