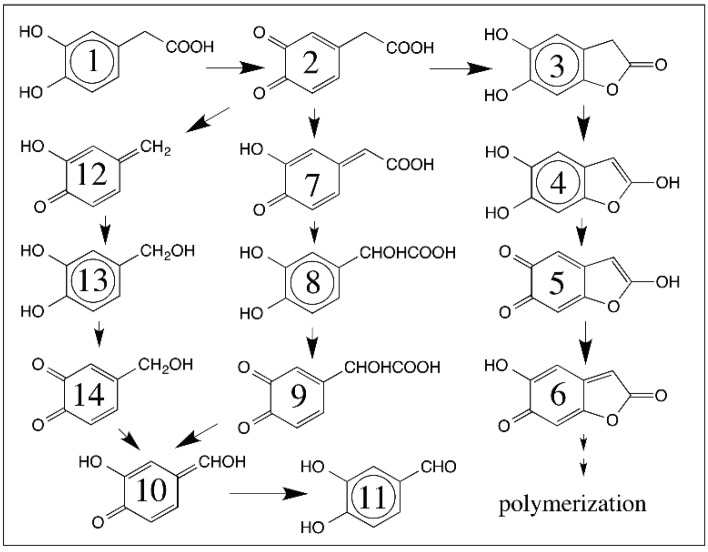
Figure 14.
Multiple routes for the oxidative transformation of 3,4-dihydroxyphenylacetic acid. 3,4-Dihydroxyphenylacetic acid (1) is readily oxidized by tyrosinase to its quinone (2). This quinone exhibits three different reactions. It can undergo decarboxylation to the quinone methide (12), which reacts with water producing 3,4-dihydroxybenylalcohol (13). 3,4-dihydroxybenylalcohol is also oxidized by tyrosinase to its quinone (14) and nonenzymatically converted to 3,4-dihydroxybenzaldehyde (11) through quinone methide intermediate (10). Carboxymethyl-o-quinone (2) also isomerizes to quinone methide (7), which generates 3,4-dihydroxymandelic acid (8) by 1,6-addtion of water. Tyrosinase catalyzed oxidation of 3,4-dihydroxy mandelic acid produces the mandeloquinone (9), that is nonenzymatically decarboxylated to the same quinone methide (10) produced by 3,4-dihydroxybenzyl alcohol, thus yielding the same final product, 3,4-dihydroxy benzaldehyde (11). Finally, quinone (2) undergoes rapid intramolecular cyclization to form 2,5,6-trihydroxy benzofuran (4) via furanone (3). This furan is initially believed to form the quinone (5) before getting converted to quinone methide (6), and eventual polymerization.