Figure 2.
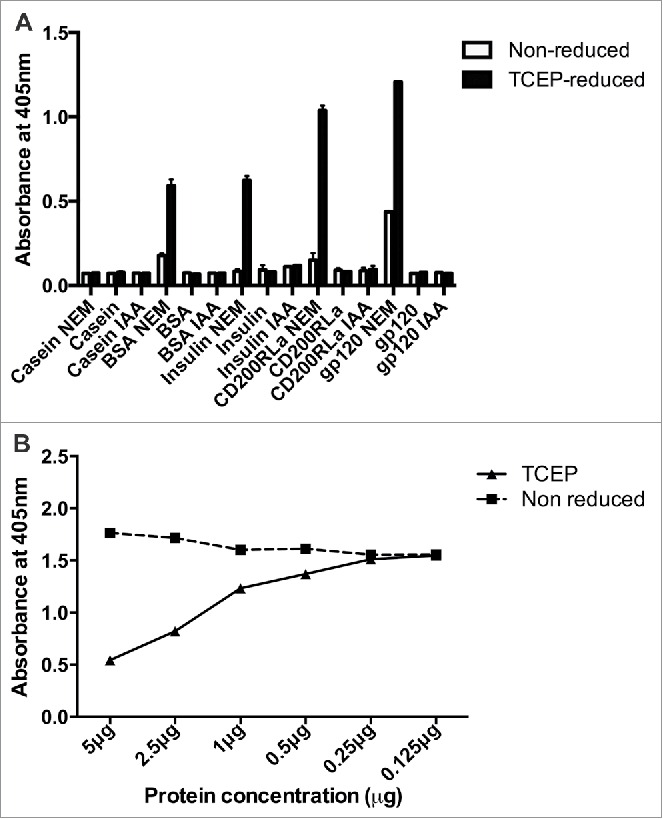
OX133 recognizes reductive changes in multiple proteins containing labile disulfide bonds (A) Target proteins (β-casein, BSA, insulin, CD200RLa or gp120 1 μg/mL) were incubated with PBS, NEM (5 mM) or IAA (5 mM) following reduction with 2.5 mM TCEP for 30 minutes or PBS (non-reduced sample) and coated onto wells of a Maxisorb microtiter plate. Wells were blocked with 0.5% (w/v) protease-free BSA in PBS/Tween for 1 hour at room temperature and alkylated reduction sites detected by incubation with OX133 antibody (0.5 μg/mL in PBS-BSA-Tween) for 1 hour at room temperature. Antibody binding to the plate was determined using anti-mouse alkaline phosphatase conjugate (1:4000) and p-NPP substrate. (B) OX133 specificity for protein-bound NEM was measured by inhibition ELISA. BSA was incubated with either PBS or TCEP (2.5 mM) for 20 minutes, then alkylated with 5 mM NEM for 30 minutes and the unreacted NEM removed. Reduced and alkylated BSA was immobilized to the plate and OX133 binding assessed in competition with free BSA or reduced and alkylated BSA. Antibody binding to the plate was determined using anti-mouse alkaline phosphatase conjugate (1:4000) and p-NPP substrate.
