Abstract
Enterotoxigenic Escherichia coli (ETEC) causes ∼20% of the acute infectious diarrhea (AID) episodes worldwide, often by producing heat-stable enterotoxins (STs), which are peptides structurally homologous to paracrine hormones of the intestinal guanylate cyclase C (GUCY2C) receptor. While molecular mechanisms mediating ST-induced intestinal secretion have been defined, advancements in therapeutics have been hampered for decades by the paucity of disease models that integrate molecular and functional endpoints amenable to high-throughput screening. Here, we reveal that mouse and human intestinal enteroids in three-dimensional ex vivo cultures express the components of the GUCY2C secretory signaling axis. ST and its structural analog, linaclotide, an FDA-approved oral secretagog, induced fluid accumulation quantified simultaneously in scores of enteroid lumens, recapitulating ETEC-induced intestinal secretion. Enteroid secretion depended on canonical molecular signaling events responsible for ETEC-induced diarrhea, including cyclic GMP (cGMP) produced by GUCY2C, activation of cGMP-dependent protein kinase (PKG), and opening of the cystic fibrosis transmembrane conductance regulator (CFTR). Importantly, pharmacological inhibition of CFTR abrogated enteroid fluid secretion, providing proof of concept for the utility of this model to screen antidiarrheal agents. Intestinal enteroids offer a unique model, integrating the GUCY2C signaling axis and luminal fluid secretion, to explore the pathophysiology of, and develop platforms for, high-throughput drug screening to identify novel compounds to prevent and treat ETEC diarrheal disease.
INTRODUCTION
Enterotoxigenic Escherichia coli (ETEC) remains a major public health issue, causing nearly 400 million illnesses and 500,000 deaths worldwide each year, with most deaths occurring in developing countries in children under age 5 years (1, 2). ETEC is a heterogeneous bacterial classification, comprising molecular subtypes of E. coli identified by their diarrhea-inducing toxins. These toxins include heat-labile enterotoxins (LT), which are structurally homologous to cholera toxin and induce cyclic AMP (cAMP) accumulation, and heat-stable enterotoxins (STa and STb), which induce cGMP accumulation (3). Of these, STa (here, simply ST) is the predominant form associated with human disease, comprising 18 amino acids containing three intrachain disulfide bonds that provide the structural stability underlying its eponymous resistance to heat-induced denaturation (3).
ST is structurally homologous to the paracrine hormones guanylin (GUCA2A) and uroguanylin (GUCA2B), which activate the intestinal guanylate cyclase C (GUCY2C) receptor (4–8). However, compared to these endogenous hormones, which contain only two disulfide bonds, ST is resistant to proteolysis, isomerically stable, pH insensitive, and has a higher receptor affinity, resulting in excess GUCY2C activation that leads to diarrhea (4–6, 9). Binding of ST to the extracellular ligand-binding domain of GUCY2C activates the cytoplasmic catalytic domain that converts GTP to cGMP (7, 10, 11). In turn, cyclic nucleotide accumulation activates cGMP-dependent protein kinase (PKG), which phosphorylates and opens the cystic fibrosis transmembrane conductance receptor (CFTR), a channel permeable to chloride and bicarbonate ions (7, 10, 12–14). Chloride ions flow down their electrochemical gradient into the intestinal lumen, resulting in electrogenic sodium flux and water secretion, which manifests as secretory diarrhea. This pathophysiological mechanism is the basis for the development of the ST analog linaclotide (Linzess), which has been approved by the FDA to treat patients with chronic constipation and constipation-type irritable bowel syndrome (15).
While prevention and therapy of ST-induced secretory diarrhea remain important priorities, available biological models limit development of effective agents (16–18). In vitro models (e.g., human colon cancer cells) may not recapitulate normal tissue architecture, biology, or molecular signaling events (13, 16, 17, 19), while in vivo models, such as suckling mice (20) and ileal loops (21), are labor-intensive and not suited to high-throughput screening. These considerations underscore the need for models of intestinal signaling and secretion which are compatible with high-throughput analyses and recapitulate the pathophysiology of ST-induced diarrhea (2, 16, 17, 22, 23).
In that context, intestinal ex vivo enteroids in culture have emerged as a transformative model of gut (patho)biology (16, 17). Stem cells isolated from mouse or human intestinal crypts are propagated in three-dimensional cultures, where they form “miniguts,” encompassing the cell types of the mature intestinal epithelium and recapitulating normal intestinal physiology (16, 17, 24). While enteroids are biologically relevant models for infectious diarrheal diseases (16, 17, 22, 23), their suitability as a platform to study ST-induced intestinal secretion remains unknown (17). Since previous studies demonstrated that pharmacological agents, including complex multimeric proteins like cholera toxin, readily access the lumen of enteroids (25, 26), we predicted that these organoids should respond to GUCY2C ligands. Here, we reveal the ability of mouse and human enteroids to respond to ST and its homologs in a GUCY2C-dependent fashion, providing a high-throughput biologically relevant model to explore the pathophysiology of ST and screen antisecretory agents to treat and prevent diarrheal disease.
MATERIALS AND METHODS
Mice.
Gucy2c+/+ and Gucy2c−/− C57BL/6 mice were bred, maintained, genotyped, and functionally characterized in the animal care facility at Thomas Jefferson University. For all experiments, mice were harvested between 6 and 12 weeks of age. The Thomas Jefferson University Institutional Animal Care and Use Committee approved all animal protocols.
Crypt isolation and enteroid culture from human small intestine.
Enteroids from normal human ileum were prepared by J. Lynch at the University of Pennsylvania. Enteroids were prepared from fresh small intestine segments from surgical cast-offs provided by the Cooperative Human Tissue Network (CHTN; https://www.chtneast.org). Since these tissues were provided without identifiers, they were deemed exempt from the requirement for IRB approval. All human enteroids used in this study were from a single individual. Ileal samples were minced, vortexed in phosphate-buffered saline (PBS) to remove debris, transferred to 30 ml of ice-cold Dulbecco's PBS (DPBS) supplemented with 8 mM EDTA and 1 mM dithiothreitol, and incubated on a rocker at 4°C for 30 min. Supernatants were discarded and replaced with 30 ml ice-cold DPBS and vortexed vigorously 10 times at 3 s per pulse, and supernatants were collected. This procedure was repeated six times, yielding 6 crypt-containing fractions. Fractions were centrifuged at 100 × g for 5 min and resuspended in 2 ml of DPBS supplemented with 10% fetal bovine serum to remove contaminating villi. Fractions were combined and centrifuged for 2 min at 100 × g, and supernatants were removed and immediately centrifuged at 120 × g for 2 min. Supernatants were removed and pellets were pooled in 5 ml of resuspension medium (advanced Dulbecco's modified Eagle's medium [DMEM]/Ham's F-12, 2 mM GlutaMAX, 10 mM HEPES, 1% penicillin-streptomycin). Pelleted crypts were resuspended in GFR matrigel matrix (Corning), and 250 crypts in 50 μl of matrigel were seeded per well in a 24-well plate and incubated at 37°C for 10 to 15 min. Cultures were then overlaid with 500 μl of complete medium (Advanced DMEM/Ham's F-12, 2 mM GlutaMAX, 10 mM HEPES, 1% penicillin-streptomycin) supplemented with 1× N-2 (Life Technologies, Carlsbad, CA), 1× B-27 (Life Technologies), 1 mM N-acetyl-l-cysteine, Wnt3a-conditioned medium (1:1; from CRL-2647 cells [ATCC]), 50 ng/ml mouse recombinant epidermal growth factor (catalog number 315-09; PeproTech, Rocky Hill, NJ), 100 ng/ml mouse recombinant Noggin (catalog number 250-38; PeproTech), 1 μg/ml human recombinant R-spondin 1 (catalog number 4645-RS-025/CF; R&D Systems), 500 nM A83-01 (Sigma), 10 μM SB202190 (catalog number S7067; Sigma), 10 nM gastrin (catalog number G9145; Sigma), and 10 mM nicotinamide (catalog number NO636; Sigma). Human enteroids were maintained at 37°C in a 5% CO2 atmosphere for at least 7 days before passaging or peptide stimulation. Enteroids from passages 1 to 3 were used for peptide stimulation assays.
Crypt isolation and enteroid culture from mouse small intestines.
Gucy2c+/+ and Gucy2c−/− mouse enteroids were established from isolated jejunal crypts; each mouse generates an average of 20,000 crypts, sufficient to seed 40 wells of enteroids. All steps were performed on ice, using reagents cooled to 4°C. Mice were euthanized with CO2, and then a 10- to 15-cm length of jejunum (beginning 3 cm distal to the stomach) was excised from the abdomen and placed in a bath of ice-cold DPBS. Using a disposable fine-tip transfer pipette, the tissue lumen was flushed with 5 ml ice-cold DPBS and then opened longitudinally. Tissue samples were flattened onto empty, chilled 10-cm petri dishes with the luminal aspect exposed. Using a glass coverslip, villi were gently scraped off and discarded. The villus-depleted jejunal tissue was then cut into pieces 3 cm in length and pooled in a 50-ml conical tube containing 30 ml of ice-cold dissociation buffer (DB; 10 mM EDTA in Hanks' balanced salt solution [HBSS]; no Ca2+, Mg2+, or phenol red). Tissues were then mechanically disrupted in a series of steps to isolate crypts from their underlying submucosa. First, samples in DB were inverted 5 times in rapid succession, and then moved to ice for 2 to 3 min to allow tissues to settle to the bottom of the tube by gravity. This cycle was repeated for a total of 70 min; every 10 min, the DB was carefully discarded and replaced with fresh DB, being careful not to discard tissue samples. Next, using a 10-ml serological pipette tip, the sample was drawn up and expelled 4 times and then allowed to settle by gravity for 3 to 5 min. After discarding the supernatant and adding 30 ml fresh DB, the sample was again pipetted up and down 5 times, and the resultant solution was passed twice through a 70-μm nylon cell strainer. Crypts in the flowthrough were then pelleted by centrifugation at 600 × g (5 min, 4°C), resuspended in 2 ml DMEM/Ham's F-12 50/50 mix (Corning), and counted. Once the total number of crypts per sample was determined, crypts were again pelleted by centrifugation and then resuspended in GFR matrigel matrix (Corning) at a final concentration of 8 to 16 intact crypts/μl. Using pipette tips stored at −20°C, 30-μl aliquots of matrigel containing a total of 500 crypts were added to a 48-well plate, and the plate was incubated at 37°C for 15 min to allow the matrigel to solidify. After matrigel solidification, 300 μl of IntestiCult OGM mouse basal medium (catalog number 06005; StemCell Technologies, Cambridge, MA) supplemented with 1% penicillin-streptomycin (Corning) was added to each well and replaced every 3 to 4 days. Mouse enteroids were maintained at 37°C in a 5% CO2 atmosphere for at least 5 days before passaging or peptide stimulation. Enteroids from passages 1 to 3 were used for peptide stimulation assays.
Secretion assays.
Established enteroids from a 5- to 7-day-old culture were seeded into a 48-well plate in 30 μl matrigel and 300 μl of IntestiCult medium, at a concentration of 250 to 500 crypts per well. One week after seeding, enteroids were incubated with various concentrations of GUCY2C-stimulating ligands (STa [catalog number 8075-S-10; Peptisyntha, Inc., Torrance, CA] or linaclotide [Ironwood Pharmaceuticals, Cambridge, MA]) or the cell-permeable cGMP homolog, 8-Br-cGMP (Sigma). For CFTR inhibition experiments, mouse and human enteroids were preincubated for 2 to 3 h with 50 μM CFTRinh-172 (Sigma), followed by addition of linaclotide to final concentrations of 1 μM and 10 μM in mouse and human enteroid cultures, respectively. To test the swelling capability of undifferentiated, crypt-like enteroids, mouse enteroids were cotreated with Wnt3a-conditioned medium (mixed 1:1 with enteroid medium) and 10 μM linaclotide. Control and stimulated enteroids were imaged using an Evos FL microscope (Life Technologies) and analyzed using ImageJ. Enteroid area was calculated using ImageJ and converted to volume {4/3π[⎷(area/π)]3}. The relative increase in volume of individual enteroids was compared to time zero measurements for each enteroid.
RNA isolation and quantitative real-time RT-PCR.
RNA was isolated from enteroid cultures by using an RNeasy RNA extraction kit (Qiagen). Relative mRNA levels of GUCY2C, GUCA2A, and GUCA2B (relative to GAPDH levels) were determined by quantitative real-time reverse transcription-PCR (RT-PCR) by using commercially available primer-probe sets (for mouse Gucy2c, Mm01267705_m1; for human GUCY2C, Hs00192035_m1; for mouse Guca2a, Mm00433863_m1; for human GUCA2A, Hs 00157859_m1; for mouse Guca2b, Mm00433865_m1; for human GUCA2B, Hs 00200071_m1; for mouse Gapdh, Mm99999915_g1; for human GAPDH, Hs02758991_g1 [Life Technologies, Carlsbad, CA]).
Immunoblot analysis.
Protein was extracted from enteroids lysed in Laemmli buffer that was supplemented with protease and phosphatase inhibitors (Roche). Lysates were analyzed by SDS-PAGE (NuPAGE 4-to-12% bis-Tris gel; Novex Life Technologies) and electrophoretically transferred to a nitrocellulose membrane (Novex Life Technologies). The membrane was blocked with 5% bovine serum albumin (BSA) in PBST (1× PBS and 1% Tween 20) and probed overnight with antibodies to the following proteins: GUCY2C (1:1000; MS20 [27]), P-VASP Ser239 (1:1,000; Cell Signaling), and GAPDH (1:2,500; Cell Signaling), followed by incubation with goat anti-mouse horseradish peroxidase (HRP)-conjugated and goat anti-rabbit HRP-conjugated secondary antibodies (1:50,000; Jackson ImmunoResearch). Blots were developed in SuperSignal West Dura enhanced chemiluminescence (ECL) substrate (Thermo Scientific). Relative intensity was quantified by densitometry using ImageJ and normalized to the instensity of GAPDH. Results reflect the average relative intensity ± standard deviation (SD) for ≥3 independent experiments with cultured enteroids per group.
Immunofluorescence.
Enteroids from 5- to 7-day-old cultures were incubated with formalin overnight at room temperature. Enteroids were washed in 70% ethanol for 10 min at room temperature and embedded in 2% agarose for histological processing and embedding in paraffin. Upon deparaffinizing and rehydration, antigens were unmasked in paraffin-embedded sections by cooking them in a pressure cooker for 15 min in Dako target retrieval buffer (pH 9.0). The antiguanylin antiserum was a gift from M. Goy (University of North Carolina) and used at a dilution of 1:100 (28); GUCY2C was stained using a monoclonal antibody (MS20 [1:1,000]) previously validated by our laboratory (29) and stained by indirect immunofluorescence (30).
Competitive cGMP ELISA.
To improve assay sensitivity and prevent cGMP degradation, enteroids were pretreated with a phosphodiesterase inhibitor (3-isobutyl-1-methylxanthine [IBMX]; 1 mM) for 1 h at 37°C (29). Since most of the cGMP generated by enteroids in response to GUCY2C activation is secreted into the medium (see Fig. S1 in the supplemental material), medium samples were tested for all cGMP quantification experiments. Following pretreatment, medium samples were collected and replaced with a fresh medium/IBMX mixture containing 10 μM ST, linaclotide, or TJU control peptide [inactive analog ST(5–17)Ala,9,17Cys(Acm),5,10 6-14 disulfide (Bachem, Bubendorf, Switzerland)] (27) and incubated for an additional 24 h at 37°C. Medium samples from control-, ST-, and linaclotide-treated enteroids and cGMP standards were subjected to acetylation prior to analysis to improve assay sensitivity, as described previously (31–33). Black Nunc-immuno 96-well plates (MaxiSorp surface) were coated overnight with 10 μg/μl goat anti-rabbit IgG (Jackson ImmunoResearch) in 0.1 M sodium carbonate, pH 9.5. Following blocking with 5% BSA in PBS, acetylated samples/standards were added to rabbit anti-cGMP serum and biotinylated cGMP and incubated overnight at 4°C with constant shaking. Biotinylated cGMP was detected with streptavidin-conjugated horseradish peroxidase (Jackson ImmunoResearch) and developed with QuantaBlu fluorogenic peroxidase substrate (Life Technologies). After 20 min, mean fluorescence intensity was quantified using an automated microplate reader (337-nm excitation, 405-nm emission). A standard curve of known cGMP concentrations was used to interpolate the concentration of cGMP in unknown samples (GraphPad Prism).
Statistical analyses.
All data were analyzed using GraphPad Prism v6. A two-way analysis of variance (ANOVA) was used to compare linaclotide-induced swelling of enteroids. Swelling results are expressed as means ± standard errors of the means (SEM). Measurements at single time points or doses were analyzed by ANOVA or Student's t test, unless otherwise indicated. Differences leading to P values of <0.05 were considered statistically significant. Error bars depict SEM unless specified. For contingency analyses, “responders” were defined as enteroids with a volume greater than the mean + 1 standard deviation, compared to enteroid volumes measured prior to treatment; “nonresponders” were defined as all other enteroids below this threshold. A chi-square test for trend was performed to determine statistical significance. Unless otherwise specified, all data represent ≥2 independent experiments, each with ≥2 replicate samples per condition.
RESULTS
The GUCA2A/B-GUCY2C axis in mouse and human enteroids.
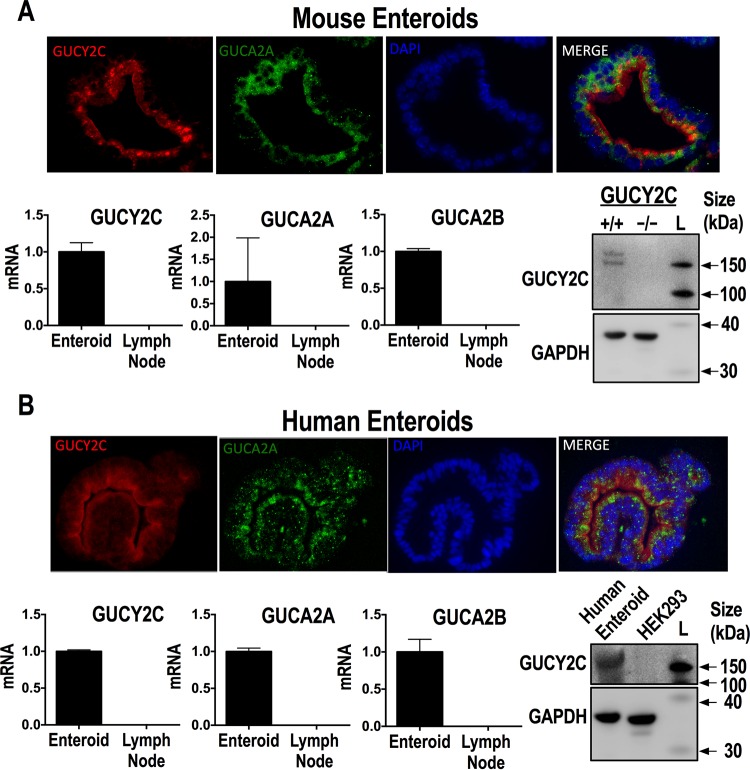
GUCY2C mRNA and protein expression were confirmed in mouse and human enteroids (Fig. 1A and B, respectively), with immunoblot analysis demonstrating multiple bands reflecting the well-established differential glycosylation pattern of GUCY2C (34). Further, immunofluorescence microscopy demonstrated GUCY2C localization in brush border membranes (Fig. 1A and B). Additionally, the GUCY2C-activating paracrine hormones guanylin (GUCA2A) and uroguanylin (GUCA2B) also were detected at the mRNA level in both mouse and human enteroids, and immunofluorescence microscopy revealed colocalization with GUCY2C (Fig. 1A and B). As a negative control for mRNA, human and mouse lymph node samples also were assessed for GUCY2C, uroguanylin, and guanylin, none of which were detected. Likewise, Gucy2c−/− mice and HEK293 cells, which do not express GUCY2C, served as negative controls for immunoblot analyses of GUCY2C protein in mouse and human enteroids, respectively (Fig. 1A and B). Together, these results indicated that the components of the GUCY2C paracrine hormone signaling axis are present in intestinal epithelial cells of established enteroids.
FIG 1.
The ST receptor GUCY2C is expressed in mouse and human enteroids. (A) Detection of GUCY2C (red) by the monoclonal antibody MS20 in wild-type mouse enteroids costained with antiguanylin antibody (green) and 4′,6-diamidino-2-phenylindole (DAPI; blue). RT-PCR mRNA quantification results (means ± SEM) are shown for GUCY2C and its endogenous peptide ligands guanylin and uroguanylin. Immunoblot analysis results are shown for GUCY2C and actin (loading control) expression in Gucy2c+/+ and Gucy2c−/− mouse enteroids; the SuperSignal molecular mass protein ladder (L; catalog number 84785; Life Technologies) was used to generate the luminescent molecular mass bands. (B) In parallel, detection of GUCY2C (red) by the monoclonal antibody MS20 in healthy human enteroids costained with antiguanylin antibody (green) and DAPI (blue) was performed. RT-PCR mRNA quantification results (means ± SEM) of GUCY2C and its endogenous peptide ligands guanylin and uroguanylin are shown. Immunoblot analysis results are shown for GUCY2C and GAPDH (loading control) expression in healthy human enteroids and HEK293 cells.
GUCY2C activation and cGMP production in enteroids.
Heat-stable enterotoxin (STa) and the ST analog linaclotide both activated GUCY2C in mouse and human enteroids, producing cGMP accumulation (Fig. 2A and B). While linaclotide induced cGMP accumulation in Gucy2c+/+ mouse enteroids, there was no effect on cyclic nucleotide accumulation in enteroids prepared from Gucy2c−/− mice (Fig. 2C). GUCY2C stimulation and cGMP accumulation activated PKG, quantified as phosphorylation of vasodilator-stimulated protein (VASP) on serine 239, a canonical downstream target of PKG, in Gucy2c+/+ mouse and human enteroids (Fig. 2D and E). While linaclotide failed to induce VASP phosphorylation in enteroids from Gucy2c−/− mice, a cell-permeable analog of the downstream effector of GUCY2C, 8Br-cGMP, induced that phosphorylation event (Fig. 2D). Thus, mouse and human enteroids maintain canonical GUCY2C signaling and cGMP-dependent activation of established downstream effectors associated with fluid secretion.
FIG 2.
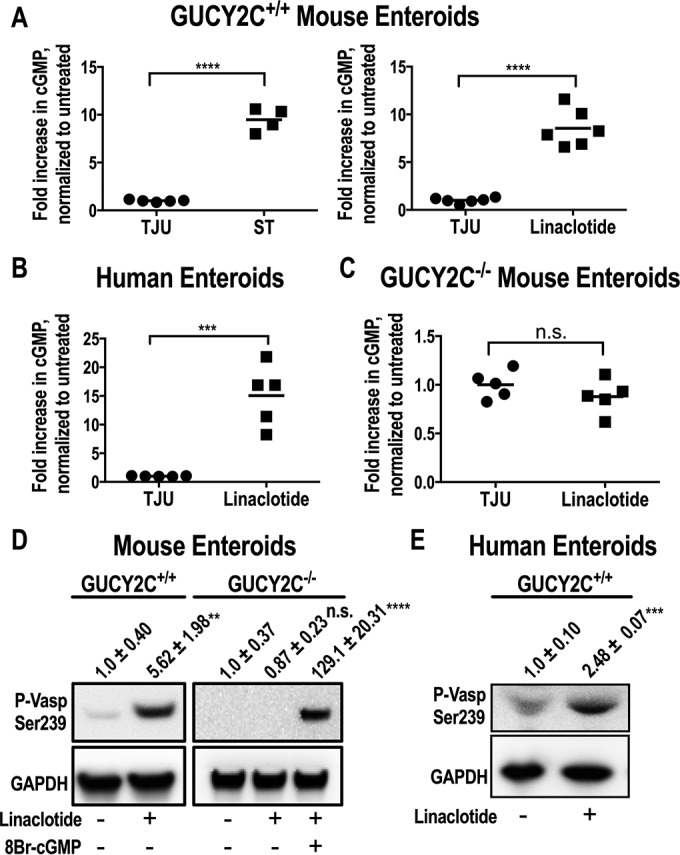
Characterization of ST-induced GUCYC2 activation and cGMP production. (A and B) cGMP production was shown in medium samples of ST-induced Gucy2c+/+ mouse enteroids (A), linaclotide-induced Gucy2c+/+ mouse enteroids (A), and healthy human enteroids (B) preincubated for 1 h with IBMX (1 mM) and stimulated with either TJU (control), ST (10 μM), or linaclotide (10 μM) for 24 h. (C) The loss of cGMP production was shown in medium samples from Gucyc−/− mouse enteroids preincubated for 1 h with IBMX (1 mM) and stimulated with either TJU (control) or linaclotide (10 μM) for 24 h. (D) GUCY2C activation was shown by immunoblot analysis of p-VASP Ser239 and GAPDH (loading control) in Gucy2c+/+ mouse enteroids treated with TJU (control) or linaclotide (1 μM) and in Gucy2c−/− mouse enteroids treated with TJU (untreated), linaclotide (1 μM), or 8-Br-cGMP (10 μM) for 24 h (results are means ± SEM). (E) Immunoblot analysis results for p-VASP Ser239 and GAPDH (loading control) in healthy human enteroids treated with TJU (control) or linaclotide (10 μM) for 24 h (results are means ± SEM). **, P < 0.01; ***, P < 0.001; ****, P < 0.0001.
ST-induced secretion in mouse and human enteroids.
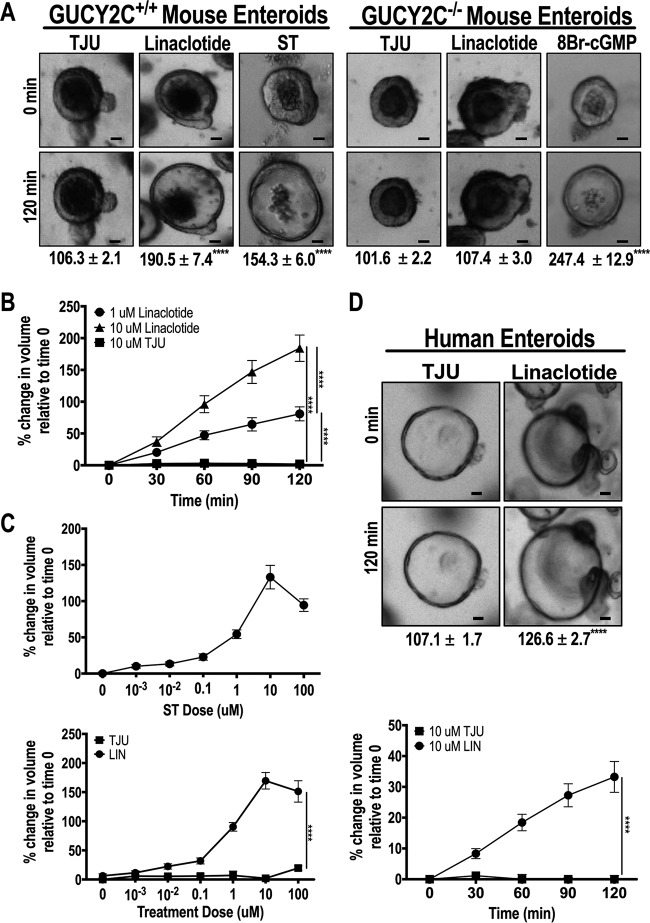
GUCY2C-induced fluid secretion requires cGMP-dependent protein kinase activation and phosphorylation of the CFTR, producing a chloride conductance triggering the secretion of fluid into the intestinal lumen (7, 10, 12–14). Three-dimensional enteroid growth results were determined in spheres of cells surrounding a central cavity analogous to the intestinal lumen, permitting quantification of secretion by measuring enteroid swelling and changes in luminal volume in real time by live-cell imaging (14, 15). ST and linaclotide, but not TJU, increased luminal volumes (Fig. 3A; see also Videos S1 and S2 in the supplemental material) in a time- and concentration-dependent fashion (Fig. 3B and C) in enteroids from Gucy2c+/+, but not Gucy2c−/−, mice (Fig. 3A; see also Videos S3 and S4 in the supplemental material). Similarly, ST and linaclotide, but not TJU, increased the fraction of secreting enteroids in a concentration-dependent fashion (see Fig. S2 in the supplemental material). While GUCY2C ligands were inactive, 8Br-cGMP increased swelling in enteroids from Gucy2c−/− mice (Fig. 3A; see also Video S5 in the supplemental material), demonstrating the competence of the signaling apparatus downstream of GUCY2C to mediate secretion in these enteroids. Similarly, linaclotide increased swelling of human enteroids in a time-dependent fashion, recapitulating GUCY2C-dependent secretion in mouse enteroids (Fig. 3D). Lastly, Wnt3a-treated mouse enteroids, which retain a cystic and crypt-like morphology due to the enrichment of Lgr5 stem cells (35), significantly increased in luminal volume when treated with linaclotide (see Fig. S3 in the supplemental material), demonstrating that Wnt3a-treated enteroids maintain GUCY2C expression and GUCY2C-dependent fluid secretion.
FIG 3.
An ST analog induces secretion in mouse and human enteroids. (A) Representative bright-field microscopy images. Bars, 30 μm. Values below images reflect quantification of swelling (means ± SEM) of Gucy2c+/+ mouse enteroids in response to TJU (control), ST (1 μM), or linaclotide (1 μM) and Gucy2c−/− mouse enteroids in response to TJU (control), linaclotide (1 μM), or 8-Br-cGMP (10 μM). (B) Gucy2c+/+ mouse enteroid swelling occurs in a time- and dose-dependent manner with linaclotide treatment (1 μM and 10 μM, respectively). (C) Normalized swelling dose responses of Gucy2c+/+ mouse enteroids after 2 h of treatment with ST (top) or linaclotide (bottom). (D) Representative bright-field microscopy images (top) and quantification of swelling (means ± SEM) (bottom) in a time-dependent manner after linaclotide treatment (10 μM) of healthy human enteroids. Bars, 30 μm. ****, P < 0.0001.
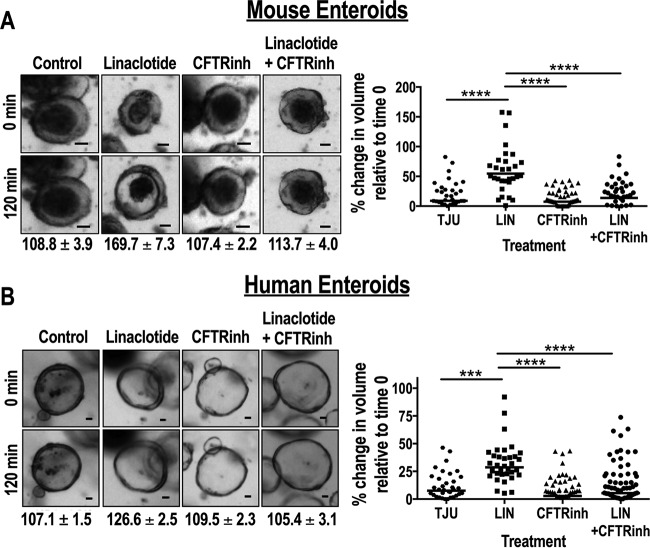
CFTR inhibition blocks GUCY2C-mediated secretion in enteroids.
GUCY2C-induced intestinal secretion reflects CFTR-dependent regulation of epithelial fluid and electrolyte transport (7, 10, 12–14, 17, 18). In that context, the CFTR has been a key target for the development of agents to prevent and treat ST-mediated secretory diarrhea (16, 18). Here, the CFTR inhibitor CFTRinh-172 (18) eliminated linaclotide-induced swelling of enteroids from mice (Fig. 4A) and humans (Fig. 4B). The data reflected the simultaneous analysis of volume changes in >50 enteroids by live-cell imaging. These studies highlight the suitability of intestinal enteroids as models for high-throughput screening of agents to prevent and treat ST-induced secretory diarrhea.
FIG 4.
ST-mediated secretion can be inhibited by CFTR inhibition, providing proof of concept for testing inhibitors of ETEC. (A) Representative bright-field microscopy images and quantification of results of a normalized swelling assay (means ± SEM) of linaclotide-induced (1 μM) Gucy2C+/+ mouse enteroids pretreated with dimethyl sulfoxide (DMSO) or CFTRinh-172 (50 μM). Bars, 30 μm. (B) In parallel, representative bright-field microscopy images and normalized swelling assay results (means ± SEM) of linaclotide-induced (10 μM) healthy human enteroids pretreated with DMSO or CFTRinh-172 (50 μM). Bars, 30 μm. ***, P < 0.001; ****, P < 0.0001. Data represent individual enteroid measurements derived from 2 to 3 independent samples, which were combined to compose the final data set.
DISCUSSION
Diarrheal disease remains one of the leading causes of morbidity and mortality, particularly in underdeveloped regions, causing 4% of all deaths and killing 1.2 million children each year (1–3). Unfortunately, there has been little progress in developing safe and effective therapies since the discovery of coupled solute and water absorption by the sodium-glucose cotransporter and the associated emergence of oral rehydration therapy more than 35 years ago (2, 22, 23). The essential molecular components, including receptors, signaling pathways, and channels regulating fluid and electrolyte fluxes in epithelial cells, have been defined (2, 16, 17, 22, 23). However, experimental models that retain the complex integration of these pathways to recapitulate net secretion and luminal accumulation of fluid volume amenable to high-throughput drug screening have been lacking (2, 16, 17, 22, 23). In vitro systems have relied on established human colorectal cancer cell lines whose genetic, genomic, and epigenetic abnormalities render them incomplete models of the (patho)physiology of secretory diarrhea (2, 17, 22, 23). Physiological in vivo models, including suckling mice and rodent intestinal loop assays, are labor-intense and low throughput, yield only semiquantitative results, and are incomplete in their interspecies generalizability, reflecting molecular differences in the regulation of fluid and electrolyte transport between rodents and humans (2, 17, 22, 23, 36).
In such a context, intestinal enteroids have emerged as a novel model that uniquely captures the structure and function of the normal intestinal epithelium. Stem cells isolated from the intestinal crypt base and propagated in three-dimensional culture ex vivo generate enterocyte, Paneth, goblet, and enteroendocrine cells that self-organize into crypt- and villus-like structures characteristic of normal intestinal mucosa (16, 24). They retain the molecular machinery required to faithfully recapitulate regulated intestinal fluid and electrolyte secretion into a central enteroid lumen (16, 17). In turn, quantification of net secretion reflects expansion of luminal volumes, measured by live cell imaging (16, 17). Enteroids are particularly amenable to high-throughput drug screening, reflecting access of macromolecules to the luminal cavity to stimulate secretion, the ability to propagate these structures from frozen stocks, and the ability to perform parallel live-image analyses of virtually unlimited numbers of enteroids simultaneously in real time (16, 17). Moreover, enteroids can be prepared from rodent or human intestine, overcoming limitations to interspecies mechanistic or therapeutic generalizability. In that context, mouse and human enteroids effectively model the pathophysiological signaling mechanisms underlying secretion induced by cholera toxin, heat-labile enterotoxins, and rotavirus and serve as a high-throughput platform to screen compounds that modulate CFTR activity (16, 17, 37).
The present studies expand earlier observations to reveal that mouse and human enteroids faithfully recapitulate the pathophysiology of fluid and electrolyte secretion induced by STs, a principle cause of secretory diarrhea in humans (1–3). STs induce secretion by binding to GUCY2C, which is expressed in apical membranes of intestinal epithelial cells from the duodenum to the distal rectum (7, 10, 11). In that context, STs reflect molecular mimicry through convergent evolution, and they are structurally homologous to the endogenous paracrine hormones uroguanylin, which is expressed in villi in the small intestine, and guanylin, which is expressed in crypts throughout the small and large intestine (4, 6, 7, 36). Their three disulfide bridges make STs resistant to proteolysis, conformationally stable, and pH resistant, resulting in greater secretory potency compared to the endogenous paracrine hormones (4–8). Like the paracrine hormones, binding of STs to the extracellular domain of GUCY2C activates the cytoplasmic catalytic domain, which converts GTP into cGMP (7, 10, 11). In turn, cGMP activates its immediate downstream effector, PKG, which phosphorylates a number of substrates, including VASP and the CFTR (7, 10, 12–14).
Here, we have demonstrated that mouse and human enteroids retain the essential components required for ST-induced pathophysiological secretion, including expression of GUCY2C, cGMP production, and PKG activation, that result in cGMP-specific VASP phosphorylation. Further, ST and its structural homolog linaclotide induce net fluid secretion, producing volume expansion of human and mouse enteroid lumens in a dose- and time-dependent fashion. Secretion is dependent on GUCY2C activation, and 8Br-cGMP, but not ST or linaclotide, induced luminal expansion in enteroids prepared from Gucy2c−/− mice. Moreover, ST-induced secretion was dependent on CFTR conductance, and an inhibitor of that channel blocked luminal expansion in enteroids induced by ST or linaclotide. It is noteworthy that STs induce net accumulation of fluid in the intestinal lumen both by inducing secretion, driven by the CFTR, and by blocking absorption, primarily by inhibiting sodium-hydrogen exchanger 3 (NHE3) (2, 7, 17, 23). Beyond the role of CFTR in fluid accumulation demonstrated here, a recent study revealed that STs regulate NHE3 in human enteroids (17).
Taken together, these observations suggest that mouse and human enteroids, and the quantification of luminal volume (16, 17), provide a mutually validating ex vivo assay system that retains the essential components required for pathophysiological intestinal secretion induced by STs. In that regard, the utility of human enteroids to model ST-induced intestinal secretion provides a unique preclinical platform to fill the existing gap in models for the development of agents that advance therapeutic paradigms in enterotoxigenic diarrhea (2, 16, 17, 22, 23). Further, the sensitivity of this system to inhibition at the level of receptors, signaling mechanisms, and channels supports its application in drug screening (2, 12, 14, 16, 17, 22, 23, 38). Moreover, the ability to quantify luminal expansion in virtually an unlimited number of enteroids, here and previously (16), suggests that this platform could be a high-throughput screening system to discover new compounds for the treatment and prevention of secretory diarrhea. It is important to note that these experiments did not test the effects of ex vivo propagation of enteroids beyond passage 3, and further studies are required to determine whether additional passages alter enteroid physiology.
Functional expression of the GUCY2C signaling axis reinforces the emerging paradigm of the utility of enteroids as a platform to explore molecular mechanisms and discover and develop therapies for infectious diarrhea (16, 17, 22, 23). Beyond ST-induced intestinal secretion, corruption of the GUCY2C paracrine hormone axis is a key step in initiating transformation in sporadic colorectal cancer (39, 40). Similarly, silencing the GUCY2C endocrine axis plays a role in the pathophysiology of obesity, while the GUCY2C paracrine axis contributes to the evolution of tumorigenesis associated with hyperphagia and morbid adiposity (30). Moreover, GUCY2C paracrine signaling defends the intestinal barrier, and its disruption contributes to inflammatory bowel disease (41–43). Expression of the complete GUCY2C hormone axis, and the recent development of enteroid platforms from transformed epithelia (44) and organoid systems integrating epithelial and mesenchymal components (45), offer a unique opportunity to couple mechanistic discovery and high-throughput drug development in ex vivo human models that address these diverse areas of an unmet therapeutic need.
Supplementary Material
ACKNOWLEDGMENTS
S.A.W. is the Chair of the Data Safety Monitoring Board for the Chart-1 trial, sponsored by Cardio3 Biosciences, and the Chair (uncompensated) of the Scientific Advisory Board of Targeted Diagnostics & Therapeutics, Inc., which provided research funding that, in part, supported this work and has a license to commercialize inventions related to this work.
Scott A. Waldman is the Samuel M. V. Hamilton Professor of Thomas Jefferson University. E.S.B. and D.J.M. received F30 Ruth Kirschstein MD-PhD Fellowship Awards.
Support for these studies was provided by grants from the National Institutes of Health (R01 CA170533 and P30 CA56036 to S.A.W., F30 DK103492-01A1 to D.J.M., and F30 CA180500 to E.S.B.); the Pennsylvania Department of Health (SAP 4100059197, SAP 4100051723); and Targeted Diagnostic and Therapeutics, Inc. (to S.A.W.).
The Pennsylvania Department of Health specifically disclaims responsibility for any analyses, interpretations, or conclusions of this study. These studies were supported by grants from NIH (R01 CA170533 to S.A.W.; U54 CA163004 and K26 OD012097 to J.P.L.) and Targeted Diagnostic and Therapeutics, Inc. (to S.A.W.).
Funding Statement
Additional support from Targeted Diagnostics and Therapeutics, Inc. (SAW).
Footnotes
Supplemental material for this article may be found at http://dx.doi.org/10.1128/IAI.00639-16.
REFERENCES
- 1.World Health Organization. 2009. Diarrhoeal diseases. World Health Organization, Geneva, Switzerland. [Google Scholar]
- 2.Donowitz M, Alpers DH, Binder HJ, Brewer T, Carrington J, Grey MJ. 2012. Translational approaches for pharmacotherapy development for acute diarrhea. Gastroenterology 142:e1–e9. doi: 10.1053/j.gastro.2012.01.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Bolin I, Wiklund G, Qadri F, Torres O, Bourgeois AL, Savarino S, Svennerholm AM. 2006. Enterotoxigenic Escherichia coli with STh and STp genotypes is associated with diarrhea both in children in areas of endemicity and in travelers. J Clin Microbiol 44:3872–3877. doi: 10.1128/JCM.00790-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Currie MG, Fok KF, Kato J, Moore RJ, Hamra FK, Duffin KL, Smith CE. 1992. Guanylin: an endogenous activator of intestinal guanylate cyclase. Proc Natl Acad Sci U S A 89:947–951. doi: 10.1073/pnas.89.3.947. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.de Sauvage FJ, Keshav S, Kuang WJ, Gillett N, Henzel W, Goeddel DV. 1992. Precursor structure, expression, and tissue distribution of human guanylin. Proc Natl Acad Sci U S A 89:9089–9093. doi: 10.1073/pnas.89.19.9089. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Kita T, Smith CE, Fok KF, Duffin KL, Moore WM, Karabatsos PJ, Kachur JF, Hamra FK, Pidhorodeckyj NV, Forte LR, et al. . 1994. Characterization of human uroguanylin: a member of the guanylin peptide family. Am J Physiol 266:F342–F348. [DOI] [PubMed] [Google Scholar]
- 7.Kuhn M. 2016. Molecular physiology of membrane guanylyl cyclase receptors. Physiol Rev 96:751–804. doi: 10.1152/physrev.00022.2015. [DOI] [PubMed] [Google Scholar]
- 8.Schulz S, Chrisman TD, Garbers DL. 1992. Cloning and expression of guanylin. Its existence in various mammalian tissues. J Biol Chem 267:16019–16021. [PubMed] [Google Scholar]
- 9.Chino N, Kubo S, Kitani T, Yoshida T, Tanabe R, Kobayashi Y, Nakazato M, Kangawa K, Kimura T. 1998. Topological isomers of human uroguanylin: interconversion between biologically active and inactive isomers. FEBS Lett 421:27–31. doi: 10.1016/S0014-5793(97)01527-5. [DOI] [PubMed] [Google Scholar]
- 10.Schulz S, Green CK, Yuen PS, Garbers DL. 1990. Guanylyl cyclase is a heat-stable enterotoxin receptor. Cell 63:941–948. doi: 10.1016/0092-8674(90)90497-3. [DOI] [PubMed] [Google Scholar]
- 11.Schulz S, Lopez MJ, Kuhn M, Garbers DL. 1997. Disruption of the guanylyl cyclase-C gene leads to a paradoxical phenotype of viable but heat-stable enterotoxin-resistant mice. J Clin Invest 100:1590–1595. doi: 10.1172/JCI119683. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Bijvelds MJ, Loos M, Bronsveld I, Hellemans A, Bongartz JP, Ver Donck L, Cox E, de Jonge HR, Schuurkes JA, De Maeyer JH. 2015. Inhibition of heat-stable toxin-induced intestinal salt and water secretion by a novel class of guanylyl cyclase C inhibitors. J Infect Dis 212:1806–1815. doi: 10.1093/infdis/jiv300. [DOI] [PubMed] [Google Scholar]
- 13.Chao AC, de Sauvage FJ, Dong YJ, Wagner JA, Goeddel DV, Gardner P. 1994. Activation of intestinal CFTR Cl− channel by heat-stable enterotoxin and guanylin via cAMP-dependent protein kinase. EMBO J 13:1065–1072. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Zhang W, Mannan I, Schulz S, Parkinson SJ, Alekseev AE, Gomez LA, Terzic A, Waldman SA. 1999. Interruption of transmembrane signaling as a novel antisecretory strategy to treat enterotoxigenic diarrhea. FASEB J 13:913–922. [DOI] [PubMed] [Google Scholar]
- 15.Mayer EA. 2008. Clinical practice. Irritable bowel syndrome. N Engl J Med 358:1692–1699. doi: 10.1056/NEJMcp0801447. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Dekkers JF, Wiegerinck CL, de Jonge HR, Bronsveld I, Janssens HM, de Winter-de Groot KM, Brandsma AM, de Jong NW, Bijvelds MJ, Scholte BJ, Nieuwenhuis EE, van den Brink S, Clevers H, van der Ent CK, Middendorp S, Beekman JM. 2013. A functional CFTR assay using primary cystic fibrosis intestinal organoids. Nat Med 19:939–945. doi: 10.1038/nm.3201. [DOI] [PubMed] [Google Scholar]
- 17.Foulke-Abel J, In J, Yin J, Zachos NC, Kovbasnjuk O, Estes MK, de Jonge H, Donowitz M. 2015. Human enteroids as a model of upper small intestinal ion transport physiology and pathophysiology. Gastroenterology 150:638–649.e8. doi: 10.1053/j.gastro.2015.11.047. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Thiagarajah JR, Verkman AS. 2012. CFTR inhibitors for treating diarrheal disease. Clin Pharmacol Ther 92:287–290. doi: 10.1038/clpt.2012.114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Sheikh A, Luo Q, Roy K, Shabaan S, Kumar P, Qadri F, Fleckenstein JM. 2014. Contribution of the highly conserved EaeH surface protein to enterotoxigenic Escherichia coli pathogenesis. Infect Immun 82:3657–3666. doi: 10.1128/IAI.01890-14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Zeng W, Azzopardi K, Hocking D, Wong CY, Robevska G, Tauschek M, Robins-Browne RM, Jackson DC. 2012. A totally synthetic lipopeptide-based self-adjuvanting vaccine induces neutralizing antibodies against heat-stable enterotoxin from enterotoxigenic Escherichia coli. Vaccine 30:4800–4806. doi: 10.1016/j.vaccine.2012.05.017. [DOI] [PubMed] [Google Scholar]
- 21.Nazarian S, Gargari SL, Rasooli I, Hasannia S, Pirooznia N. 2014. A PLGA-encapsulated chimeric protein protects against adherence and toxicity of enterotoxigenic Escherichia coli. Microbiol Res 169:205–212. doi: 10.1016/j.micres.2013.06.005. [DOI] [PubMed] [Google Scholar]
- 22.Kovbasnjuk O, Zachos NC, In J, Foulke-Abel J, Ettayebi K, Hyser JM, Broughman JR, Zeng XL, Middendorp S, de Jonge HR, Estes MK, Donowitz M. 2013. Human enteroids: preclinical models of non-inflammatory diarrhea. Stem Cell Res Ther 4(Suppl 1):S3. doi: 10.1186/scrt364. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Zachos NC, Kovbasnjuk O, Foulke-Abel J, In J, Blutt SE, de Jonge HR, Estes MK, Donowitz M. 2016. Human enteroids/colonoids and intestinal organoids functionally recapitulate normal intestinal physiology and pathophysiology. J Biol Chem 291:3759–3766. doi: 10.1074/jbc.R114.635995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Sato T, Clevers H. 2013. Growing self-organizing mini-guts from a single intestinal stem cell: mechanism and applications. Science 340:1190–1194. doi: 10.1126/science.1234852. [DOI] [PubMed] [Google Scholar]
- 25.Zomer-van Ommen DD, Pukin AV, Fu O, Quarles van Ufford LH, Janssens HM, Beekman JM, Pieters RJ. 2016. Functional characterization of cholera toxin inhibitors using human intestinal organoids. J Med Chem 59:6968–6972. doi: 10.1021/acs.jmedchem.6b00770. [DOI] [PubMed] [Google Scholar]
- 26.Foulke-Abel J, In J, Yin J, Zachos NC, Kovbasnjuk O, Estes MK, de Jonge H, Donowitz M. 2016. Human enteroids as a model of upper small intestinal ion transport physiology and pathophysiology. Gastroenterology 150:638–649. doi: 10.1053/j.gastro.2015.11.047. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Valentino MA, Lin JE, Snook AE, Li P, Kim GW, Marszalowicz G, Magee MS, Hyslop T, Schulz S, Waldman SA. 2011. A uroguanylin-GUCY2C endocrine axis regulates feeding in mice. J Clin Invest 121:3578–3588. doi: 10.1172/JCI57925. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Li Z, Taylor-Blake B, Light AR, Goy MF. 1995. Guanylin, an endogenous ligand for C-type guanylate cyclase, is produced by goblet cells in the rat intestine. Gastroenterology 109:1863–1875. doi: 10.1016/0016-5085(95)90753-X. [DOI] [PubMed] [Google Scholar]
- 29.Marszalowicz GP, Snook AE, Magee MS, Merlino D, Berman-Booty LD, Waldman SA. 2014. GUCY2C lysosomotropic endocytosis delivers immunotoxin therapy to metastatic colorectal cancer. Oncotarget 5:9460–9471. doi: 10.18632/oncotarget.2455. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Lin JE, Colon-Gonzalez F, Blomain E, Kim GW, Aing A, Stoecker B, Rock J, Snook AE, Zhan T, Hyslop TM, Tomczak M, Blumberg RS, Waldman SA. 2016. Obesity-induced colorectal cancer is driven by caloric silencing of the guanylin-GUCY2C paracrine signaling axis. Cancer Res 76:339–346. doi: 10.1158/0008-5472.CAN-15-1467-T. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Murad F, Waldman SA, Fiscus RR, Rapoport RM. 1986. Regulation of cyclic GMP synthesis and the interactions with calcium. J Cardiovasc Pharmacol 8(Suppl 8):S57–S60. [DOI] [PubMed] [Google Scholar]
- 32.Goldberg ML. 1977. Radioimmunoassay for adenosine 3′,5′-cyclic monophosphate and guanosine 3′,5′-cyclic monophosphate in human blood, urine, and cerebrospinal fluid. Clin Chem 23:576–580. [PubMed] [Google Scholar]
- 33.Frandsen EK, Krishna G. 1976. A simple ultrasensitive method for the assay of cyclic AMP and cyclic GMP in tissues. Life Sci 18:529–541. doi: 10.1016/0024-3205(76)90331-3. [DOI] [PubMed] [Google Scholar]
- 34.Ghanekar Y, Chandrashaker A, Tatu U, Visweswariah SS. 2004. Glycosylation of the receptor guanylate cyclase C: role in ligand binding and catalytic activity. Biochem J 379:653–663. doi: 10.1042/bj20040001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Farin HF, Van Es JH, Clevers H. 2012. Redundant sources of Wnt regulate intestinal stem cells and promote formation of Paneth cells. Gastroenterology 143:1518–1529. doi: 10.1053/j.gastro.2012.08.031. [DOI] [PubMed] [Google Scholar]
- 36.Brenna O, Furnes MW, Munkvold B, Kidd M, Sandvik AK, Gustafsson BI. 2016. Cellular localization of guanylin and uroguanylin mRNAs in human and rat duodenal and colonic mucosa. Cell Tissue Res 365:331–341. doi: 10.1007/s00441-016-2393-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Finkbeiner SR, Zeng XL, Utama B, Atmar RL, Shroyer NF, Estes MK. 2012. Stem cell-derived human intestinal organoids as an infection model for rotaviruses. mBio 3(4):e00159-12. doi: 10.1128/mBio.00159-12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Tian X, Michal AM, Li P, Wolfe HR, Waldman SA, Wickstrom E. 2008. STa peptide analogs for probing guanylyl cyclase C Biopolymers 90:713–723. doi: 10.1002/bip.21045. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Blomain E, Pattison A, Waldman SA. 2016. GUCY2C ligand replacement to prevent colorectal cancer. Cancer Biol Ther 17:713–718. doi: 10.1080/15384047.2016.1178429. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Shailubhai K, Palejwala V, Arjunan KP, Saykhedkar S, Nefsky B, Foss JA, Comiskey S, Jacob GS, Plevy SE. 2015. Plecanatide and dolcanatide, novel guanylate cyclase-C agonists, ameliorate gastrointestinal inflammation in experimental models of murine colitis. World J Gastrointest Pharmacol Ther 6:213–222. doi: 10.4292/wjgpt.v6.i4.213. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Brenna O, Bruland T, Furnes MW, Granlund A, Drozdov I, Emgard J, Bronstad G, Kidd M, Sandvik AK, Gustafsson BI. 2015. The guanylate cyclase-C signaling pathway is down-regulated in inflammatory bowel disease. Scand J Gastroenterol 50:1241–1252. doi: 10.3109/00365521.2015.1038849. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Han X, Mann E, Gilbert S, Guan Y, Steinbrecher KA, Montrose MH, Cohen MB. 2011. Loss of guanylyl cyclase C (GCC) signaling leads to dysfunctional intestinal barrier. PLoS One 6:e16139. doi: 10.1371/journal.pone.0016139. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Lin JE, Snook AE, Li P, Stoecker BA, Kim GW, Magee MS, Garcia AV, Valentino MA, Hyslop T, Schulz S, Waldman SA. 2012. GUCY2C opposes systemic genotoxic tumorigenesis by regulating AKT-dependent intestinal barrier integrity. PLoS One 7:e31686. doi: 10.1371/journal.pone.0031686. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Weeber F, van de Wetering M, Hoogstraat M, Dijkstra KK, Krijgsman O, Kuilman T, Gadellaa-van Hooijdonk CG, van der Velden DL, Peeper DS, Cuppen EP, Vries RG, Clevers H, Voest EE. 2015. Preserved genetic diversity in organoids cultured from biopsies of human colorectal cancer metastases. Proc Natl Acad Sci U S A 112:13308–13311. doi: 10.1073/pnas.1516689112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Spence JR, Mayhew CN, Rankin SA, Kuhar MF, Vallance JE, Tolle K, Hoskins EE, Kalinichenko VV, Wells SI, Zorn AM, Shroyer NF, Wells JM. 2011. Directed differentiation of human pluripotent stem cells into intestinal tissue in vitro. Nature 470:105–109. doi: 10.1038/nature09691. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.