Abstract
Background:
Oral isotretinoin is highly effective in all forms and grades of acne, even in lower dosages (<0.5 mg/kg/day). There is a paucity of comparative data on the various low-dose regimens of oral isotretinoin in the Indian literature.
Objectives:
To assess and compare the efficacy and tolerability of two low-dose oral isotretinoin treatment regimens (20 mg daily and 20 mg alternate days) in moderate to severe acne vulgaris.
Materials and Methods:
A total of 240 patients with moderate to severe acne vulgaris were selected and randomized into two groups and treated with a fixed dose of 20 mg of isotretinoin (Group A - daily and Group B - alternate days) for 24 weeks and followed up for 12 weeks post therapy.
Results:
A total of 234 patients completed the study. At the end of therapy, decrease in the total acne loads up to 98.99% (Group A) and 97.69% (Group B) was achieved from the baseline (P < 0.01), excellent response was observed in 98.3% (Group A) and 93.96% (Group B) patients (P = 0.166). In the severe acne, Group A performed significantly better than Group B until the end of 36 weeks. While in the moderate acne, significant difference in the response between both groups was observed only up to 12 weeks. No serious side effect was observed.
Conclusion:
Both isotretinoin regimens were well tolerated and found to be an effective treatment for moderate to severe acne vulgaris. However, in moderate acne 20 mg alternate day regimen may be preferred. A 20 mg daily regimen is a better choice for severe acne in terms of response.
Limitation:
Small sample size and short follow-up period.
Keywords: Acne vulgaris, fixed dose, low-dose isotretinoin
INTRODUCTION
Acne vulgaris is a common inflammatory disease of the pilosebaceous unit,[1] which affects up to 87% adolescence and 54% of adults with varying degrees of severity.[2,3] Left untreated or inadequately treated, acne vulgaris can lead to psychological and physical scarring.[4,5] Treatment improves the QOL of patients with acne and can prevent scarring.[6] According to severity of acne, there are various topical and systemic treatment modalities. In systemic therapy, the commonly used drugs are oral antibiotics, isotretinoin, and hormones.
The multiple modes of action of isotretinoin (13-cis-retinoic acid) makes this compound the major pharmacological breakthrough in acne therapeutics.[7] Over the time, oral isotretinoin has proven to be a “wonder drug” that is highly effective in the treatment of all forms and grades of acne vulgaris, even in lower dosages.[8]
Oral isotretinoin in the standard regimen of 0.5–1.0 mg/kg/day for 16–32 weeks causes many dose-dependent mucocutaneous and systemic adverse effects. Various studies have reinforced the view that lower doses of isotretinoin are also effective in terms of response, adverse effects, and cost; therefore, other regimens should be used instead of the daily standard regimen. There are few randomized comparative studies of low-dose regimens of oral isotretinoin in the Indian literature. Thus, the present study was undertaken to compare the efficacy and tolerability of two different fixed low-dose oral isotretinoin regimens in moderate to severe acne vulgaris with a lager sample size and a longer follow-up period.
Aims and objectives
To assess and compare the efficacy and tolerability of two fixed low-dose (20 mg daily and alternate day) oral isotretinoin regimens in moderate to severe acne vulgaris.
MATERIALS AND METHODS
This prospective randomized comparative study included 240 patients with moderate to severe acne vulgaris attending the outpatient clinic in the dermatology department. Patients with a personal or family history of hyperlipidemia or diabetes, and those having drug-induced acne were excluded. Pregnant women, women desiring pregnancy, and women using temporary methods of contraception were also excluded. Acne involving all body sites was studied. A written consent was taken from the patients or their parents. After recording detailed demographic data, the patients were examined under good illumination. Patients were finally graded into mild, moderate, and severe acne based on severity as described by Pochi [Table 1].[9] To enable analysis of improvement in lesion count, total acne load (TAL) was calculated with the help of Definition Severity index [Table 2].[10] The patients were randomly assigned into two groups (A and B); each group consisted of 120 patients. Groups A and B were treated with a fixed dose of 20 mg of oral isotretinoin daily and alternate days, respectively, for a total period of 24 weeks and followed up for 12 weeks post-therapy. Patients were also advised to apply topical 1% clindamycin gel twice daily and white petroleum jelly on lips when required. All patients visited at an interval of two weeks for 24 weeks then six weekly interval for 12 weeks after completion of treatment. The adverse effects and response according to the number and types of lesions were recorded at every visit. TAL was also calculated at each visit. Complete blood cell counts, liver function tests, and serum lipid profile were done initially and repeated at four and eight weeks thereafter. Emergence of near pretreatment severity of acne in the treated patient within 12 weeks of follow-up was considered as relapse. Greater than twofold increase in baseline laboratory values of liver function tests and serum lipid profile was considered a criterion for discontinuation of therapy. Treatment response was evaluated according to mean percentage decrease in TAL. Treatment response was also evaluated according to response criteria, which is as follows:
Table 1.
Pochi et al. criteria for grading of acne vulgaris

Table 2.
Definition severity index

1+ = Poor response (<30% reduction in the lesion counts)
2+ = Fair response (30%–60% reduction in the lesion counts)
3+ = Good response (60%–90% reduction in the lesion counts)
4+ = Excellent response (>90% reduction in the lesion counts).
Statistical analyses were done using computer software (SPSS version 20 and primer). All the findings were analyzed by using Chi-square test, Student's t-test and one-way analysis of variance (ANOVA), Mann–Whitney U-test, repeated ANOVA, and Wilcoxon-statistical test wherever required. Significance level for tests was determined as 95% (P < 0.05). The study protocol was approved by the research review board of the institution and had no financial support from any outside agency.
RESULTS
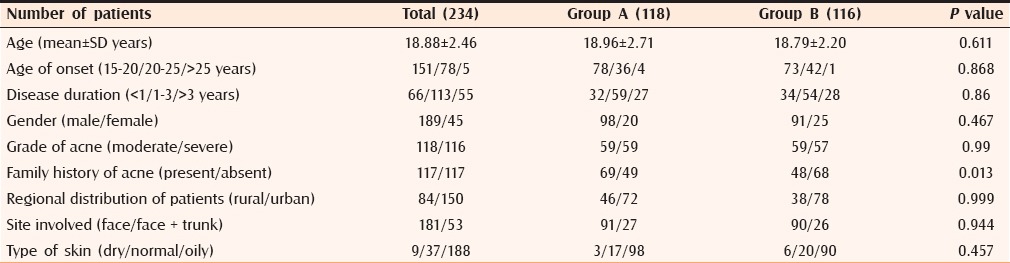
In the present study, a total of 240 patients with a mean age of 18.88 ± 2.46 years (range 15–30; median 18.51) were selected prospectively and randomly assigned into two groups. Out of 240 patients, six patients were lost to follow up during the study period. For final result analysis, there were 234 patients (Group A, 118 and Group B, 116). No statistically significant difference was observed in age, gender, and disease characteristics between the two groups [Table 3]. A statistically significant difference was observed in the family history of acne among both groups.
Table 3.
Patient characteristics
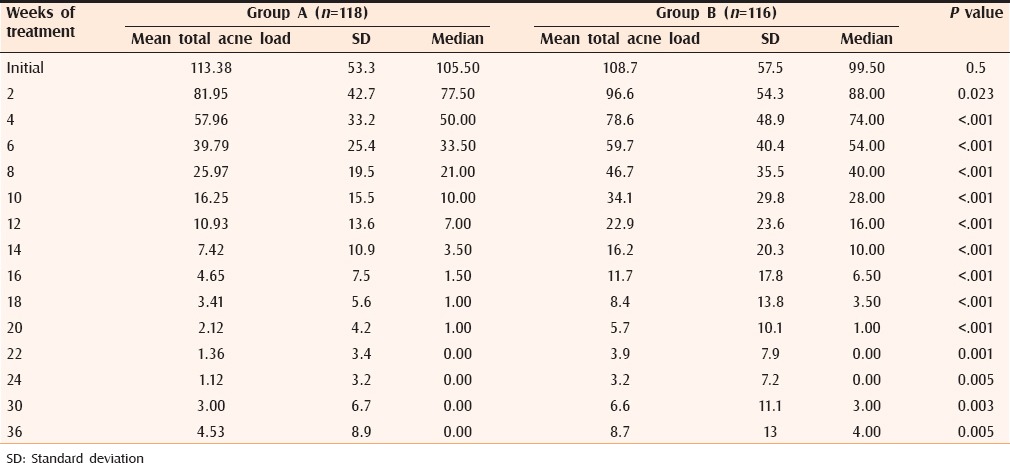
The initial mean TAL in both groups was comparable as per Mann–Whitney tests for independent samples (Group A = 113.38 ± 53.3; Group B = 108.7 ± 57.5; P value > 0.5). During follow-up, a statistically significant decrease in TAL in both groups was observed as per Wilcoxon paired two-tailed probability test (P value < 0.001 at each follow up) [Table 4].
Table 4.
Mean total acne load at different time intervals in both groups
Response rates according to mean percentage decrease in TAL in both the groups are summarized in Table 5 and Figure 1. On application of ANOVA with repeated measures, Group A was found to perform significantly better in terms of mean percentage decrease in TAL as compared with Group B during the whole study period (P value < 0.01 at each follow up).
Table 5.
Mean percentage decrease in total acne load at different time intervals in both groups
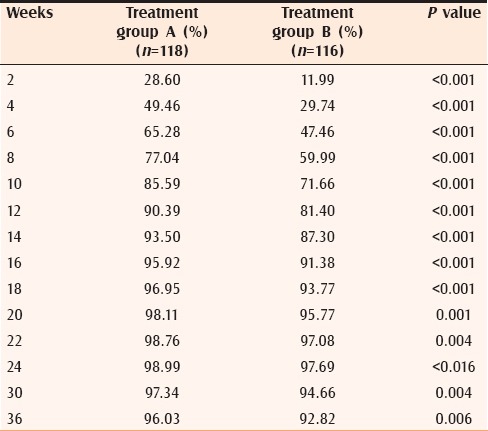
Figure 1.
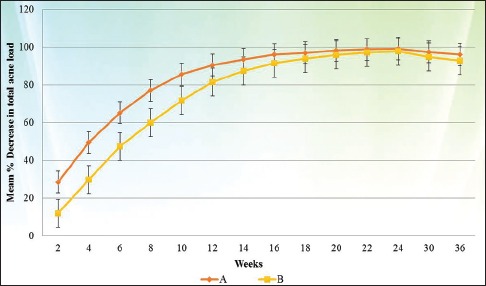
Response according to mean percentage decrease in total acne load at different time interval
When the results were evaluated according to severity of acne, in cases of severe acne a statistically significant difference in response rate was noted between both groups (Group A better than Group B) during the whole study period (P value < 0.005 at each follow up) [Figure 2], whereas in cases of moderate acne statistically significant difference between both groups (Group A better than Group B) was observed only up to 12 weeks (P value < 0.02) [Figure 3].
Figure 2.
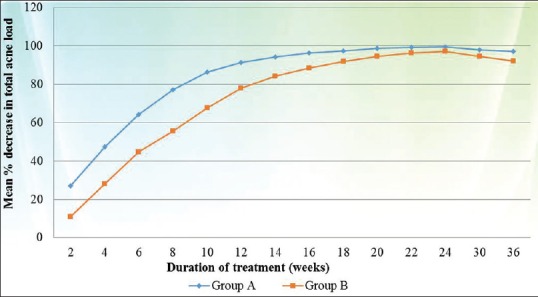
Comparison of mean percentage decrease in TAL between both groups in the cases of severe acne
Figure 3.
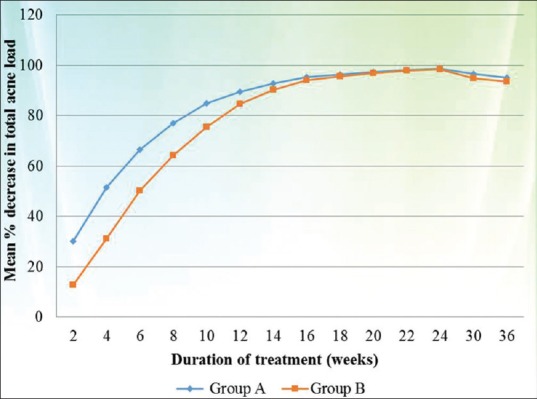
Comparison of mean percentage decrease in TAL between both groups in the cases of moderate acne
According to response criteria, a statistically significant difference in the response was observed between both groups (Group A better than Group B) during the whole study period, whereas at the end of therapy (24 weeks) the response was same in both groups. At the end of 24 weeks, the excellent response in treatment groups A and B was seen in 98.3% and 93.96% patients, respectively (P 0.166), which at the end of 36 weeks decreased to 90.6% and 74.1% patients, respectively (P 0.004) (Figures 4–6: clinical photographs).
Figure 4.
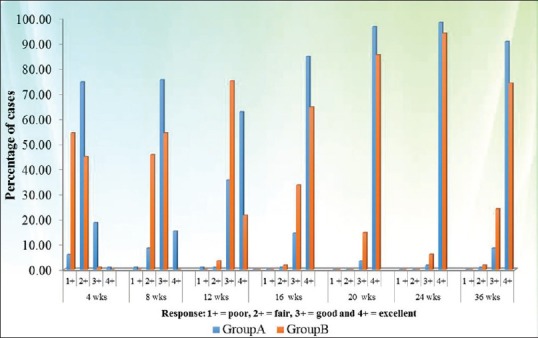
Comparison of response according to response criteria at different time intervals in both groups
Figure 6.
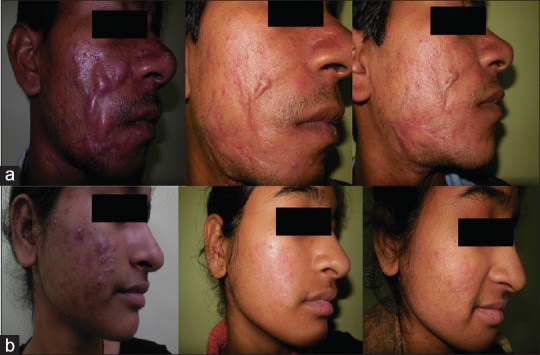
Clinical photographs of Group B patients: (a) Severe acne at 0, 24, and 36 weeks (b) Moderate acne at 0, 24, and 36 weeks
Figure 5.
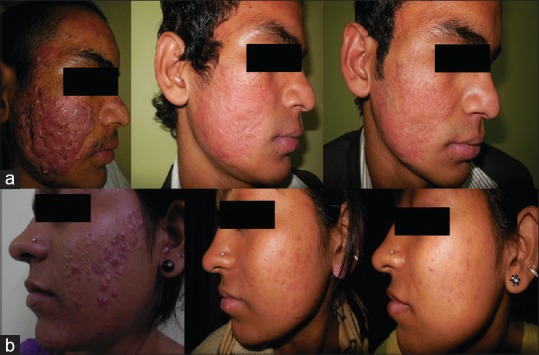
Clinical photographs of Group A patients: (a) Severe acne at 0, 24, and 36 weeks (b) Moderate acne at 0, 24, and 36 weeks
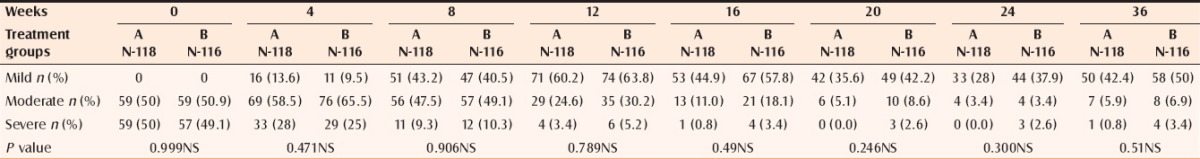
At 24 weeks 68.64% (Group A) and 56.03% (Group B) patients were acne free, which at the end of 36 weeks decreased to 50.84% and 39.65%, respectively (P 0.51) [Table 6].
Table 6.
Percentage of the cases with various severities of acne at different time intervals
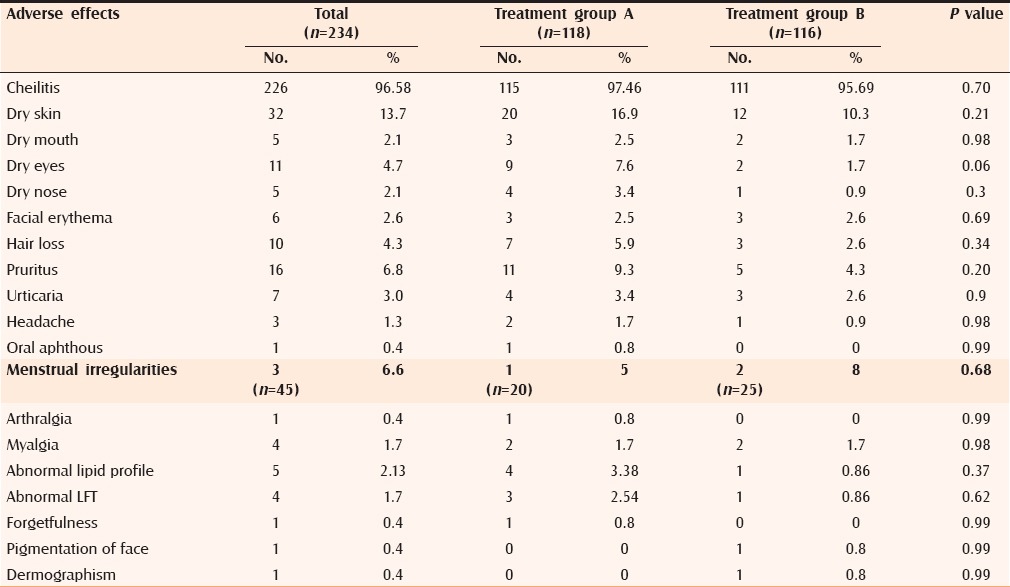
Most common adverse effects observed were cheilitis, dry skin, pruritus, dry eyes, hair fall, and other less-frequent adverse effects were also observed that are shown in Table 7. Although the frequency of adverse effects was slightly higher in Group A as compared with Group B, the difference was not statistically significant (P > 0.05).
Table 7.
Frequency of observed adverse effects
DISCUSSION
In the era of smart phones with increasing popularity of social media and exchange of selfies between adolescents and young adults, the predominance of acne vulgaris in this psychologically labile age group coupled with its potentially lifelong sequelae makes it a matter of great financial and psychosocial concern. Oral isotertinoin continues to be the mainstay of therapy for acne, because of its efficacy, ability to target all the four pathophysiologic factors, high rates of permanent remission, and prevention of permanent scarring in acne.[11,12,13,14] Multiple studies have addressed that the duration of remission after initial course of isotretinoin is variable and appear to correlate with several potential contributory factors, for example, age of patient, male gender, cumulative dose administered, endogenous androgen-excess (ie, polycystic ovary syndrome), presence/persistence of macrocomedones, presence of sinus tracts, and patient adherence.[15] Recurrence of acne after an initial course of isotretinoin is not always of the same severity as before isotretinoin treatment.[16] Although isotretinoin was approved by the FDA for treatment of severe recalcitrant nodular acne, the appropriate use of isotretinoin also includes patients with moderate-to-severe acne or lesser degree of acne producing physical scarring or psychological distress and unresponsive to adequate conventional therapy.[17] It has been suggested that isotretinoin should be initiated early in the management of acne, even lower-dose isotretinoin (0.25–0.5 mg/kg/day for 24 weeks) offering a good balance between efficacy and dose-related adverse effects.[8,18] To decrease the incidence of adverse effects and to increase adherence of patients to therapy, the different low-dose isotretinoin regimens for different duration has been tried to treat acne in various studies (summarized in Table 8). The doses of isotretinoin that were used in these studies varied from 0.14 to 0.75 mg/kg/day. The cumulative dosage varied from 21 mg/kg to as high as 180 mg/kg with a mean dose of 49.71 mg/kg. On comparison of these studies, the efficacy of low-dose isotretinoin in the treatment of acne varied from 69% to 99.8% and the relapse rate varied from 0% to 39%.
Table 8.
Comparison of different low-dose isotretinoin studies
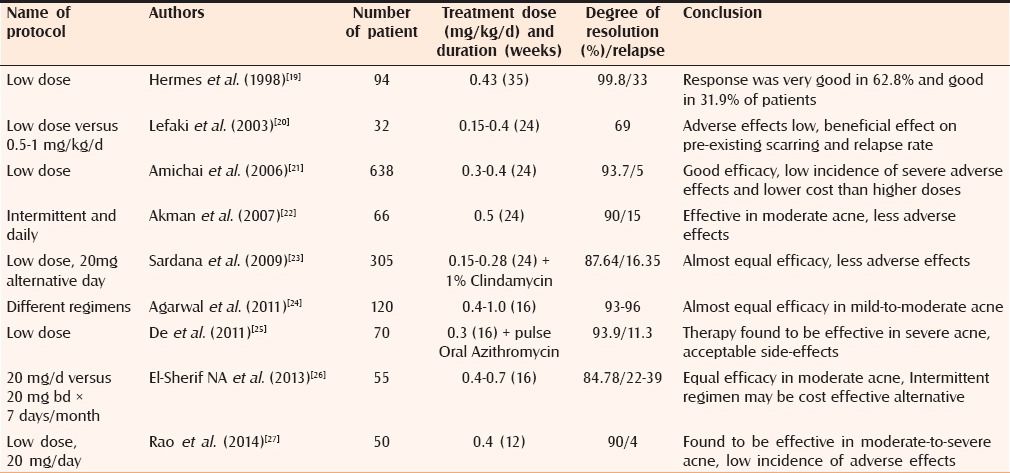
Akman assessed the efficacy 0.5 mg/kg/day oral isotretinoin (10 days/month × six months or each day in first month then 10 days/month × five months or daily × six months) in moderate-to-severe acne and reported 90% resolution and relapse in 15% of cases.
Sardana et al. studied the efficacy of low-fixed dose isotretinoin (0.15–0.28 mg/kg/d × six months) plus topical 1% clindamycin gel in moderate grade of acne and observed clinically significant results in 87.54% of the patients, including 68.20% “very good” and 19.34% of “good” results.
In a recent study by Rao et al. (20 mg/d for a period of three months in moderate-to-severe acne), reported very good results in 90% of participants and relapse in 4% of participants.
Most of these studies have found that different low-dose regimens of isotretinoin are effective in moderate-to-severe acne with a low incidence and severity of adverse effects.
In the present study, we have tried to compare the response of two different fixed low-dose regimens of oral isotretinoin in moderate to severe acne vulgaris. We have tried to correlate treatment response on the basis of various scales such as mean percentage decrease in TAL, response criteria, and disease severity. A significant reduction in mean TAL from the initial mean TAL was observed in the both groups. The initial mean TAL in groups A and B was 113.3 ± 53.3 and 108.7 ± 57.5, respectively, which at the end of therapy at 24 weeks decreased to 1.12 ± 3.2 and 3.2 ± 7.2, respectively. We observed that Group A performed significantly better as compared with Group B during the whole study period. The mean percentage decrease in TAL in Group A and Group B at 24 weeks was 98.99% and 97.69%, respectively ((P value < 0.01), which decreased to 96.03% and 92.82%, respectively, at 36 weeks ((P value < 0.01). When both the groups were compared according to severity of acne, in the cases of severe acne significant difference was observed in terms of response between groups A and B until the end of 36 weeks. While in the cases of moderate acne, significant difference in the response between both groups was observed only up to 12 weeks.
According to response criteria, at 24 weeks the excellent response in groups A and B was observed in 98.3% and 93.96% patients, respectively (P 0.166) which at 36 weeks decreased to 90.6% and 74.1% patients, respectively (P 0.004).
Although our study found that number of acne-free patients seen at the end of therapy was decreased at the end of follow up; however, the patients developing acne recurrence had mild-grade acne.
The doses of isotretinoin used in the present study were in the range of doses used in previous studies; however, the degree of improvement observed during our study was higher than most of the previous similar low-dose isotretinoin studies, this may be due to either the use of topical 1% clindamycin phosphate gel as adjuvant or because of the longer treatment period in our study.
In the present study, both treatment regimens were well tolerated. Most common adverse effects noted in both groups were cheilitis (Group A, 97.46% and Group B, 95.69%) and dry skin (Group A, -16.9% and Group B, 10.3%). Other less frequent adverse effects observed were pruritus, dry eyes, hair fall, urticaria, dry mouth, dry nose, facial redness on sun exposure, menstrual irregularities, head ache, myalgia, arthralgia, oral aphthous, moderately increased triglycerides level, abnormal liver function test, forgetfulness, dermographism, and pigmentation of face. All the adverse effects were managed successfully; none of the patients required discontinuation of therapy because of adverse effects. Various previous studies also showed that low-dose isotretinoin has lesser adverse effects as compared with the conventional high-dose regimen. None of our patients in either group reported initial flare of their acne. Borghi et al. reported that isotretinoin in the doses of < 0.2 mg/kg reduces the risk of acne flare upon initiation of therapy.[28]
Various studies have reported many neuropsychiatric adverse effects.[29] However, in our study one patient developed forgetfulness, which was reported in none of the previous low-dose studies. In 1983, in a series, 6/110 (5.5%) of isotretinoin-treated patients (1–2 mg/kg/day) developed symptoms of depressed mood, crying spells, malaise, and forgetfulness.[30] In contrast to various previous studies of low-dose isotretinoin, none of the patients in our study showed relapse, which may be due to the short follow-up period of 12 weeks.
CONCLUSION
We conclude that fixed low-dose regimen of oral isotretinoin should be encouraged because of excellent response in moderate-to-severe acne with the advantage of lesser adverse effects, patient compliance, and cost effectiveness. In moderate acne 20 mg alternate day regimen can be preferred, but for severe acne 20 mg daily regimen is a better choice in the terms of response. Limitations of the current study were a small sample size and short follow-up period. Prospective studies with larger sample sizes and longer durations of follow up are therefore required.
Declaration of patient consent
The authors certify that they have obtained all appropriate patient consent forms. In the form the patient(s) has/have given his/her/their consent for his/her/their images and other clinical information to be reported in the journal. The patients understand that their names and initials will not be published and due efforts will be made to conceal their identity, but anonymity cannot be guaranteed.
Financial support and sponsorship
Nil.
Conflicts of interest
There are no conflicts of interest.
REFERENCES
- 1.Dawson AL, Dellavalle RP. Acne vulgaris. BMJ. 2013;346:2634. doi: 10.1136/bmj.f2634. [DOI] [PubMed] [Google Scholar]
- 2.Dreno B, Poli F. Epidemiology of acne. Dermatology. 2003;206:7–10. doi: 10.1159/000067817. [DOI] [PubMed] [Google Scholar]
- 3.Goulden V, Stables GI, Cunliffe WJ. Prevalence of facial acne in adults. J Am Acad Dermatol. 1999;41:577–80. [PubMed] [Google Scholar]
- 4.Layton AM. Acne scarring: Reviewing the need for early treatment of acne. J Dermatol Treat. 2000;11:3–6. [Google Scholar]
- 5.Fried RG, Webster GF, Eichenfield LF, Friedlander SF, Fowler JF, Jr, Levy ML. Medical and psychosocial impact of acne. Semin Cutan Med Surg. 2010;29(Suppl 1):9–12. doi: 10.1016/j.sder.2010.04.004. [DOI] [PubMed] [Google Scholar]
- 6.Fried R, Nighland M. Acne quality of life and patient satisfaction following treatment with tretinoin pump. J Drugs Dermatol. 2009;8:1080–5. [PubMed] [Google Scholar]
- 7.Shahidullah M, Tham SN, Goh CL. Isotretinoin therapy in acne vulgaris: A 10-year retrospective study in Singapore. Int J Dermatol. 1994;33:60–3. doi: 10.1111/j.1365-4362.1994.tb01500.x. [DOI] [PubMed] [Google Scholar]
- 8.Lee JW, Yoo KH, Park KY, Han TY, Li K, Seo SJ, et al. Effectiveness of conventional, low-dose and intermittent oral isotretinoin in the treatment of acne: A randomized, controlled comparative study. Br J Dermatol. 2011;164:1369–75. doi: 10.1111/j.1365-2133.2010.10152.x. [DOI] [PubMed] [Google Scholar]
- 9.Pochi PE, Shalita AR, Strauss JS, Webster SB, Cunliffe WJ, Katz HI, et al. Report of the Consensus Conference on Acne Classification. Washington, D.C., March 24 and 25, 1990. J Am Acad Dermatol. 1991;24:495–500. doi: 10.1016/s0190-9622(08)80076-x. [DOI] [PubMed] [Google Scholar]
- 10.Lidén S, Göransson K, Odsell L. Clinical evaluation in acne. Acta Dermato Venereol Suppl (Stockh) 1980;89(Suppl):47–52. [PubMed] [Google Scholar]
- 11.Patton TJ, Ferris LK. Systemic retinoids. In: Wolverton SE, editor. Comprehensive Dermatologic Drug Therapy. 3rd ed. Edinburg: Elsevier-Saunders; 2013. pp. 252–68. [Google Scholar]
- 12.Peck GL, Olsen TG, Yoder FW, Strauss JS, Downing DT, Pandya M, et al. Prolonged remissions of cystic and conglobate acne with 13-cis-retinoic acid. N Engl J Med. 1979;300:329–33. doi: 10.1056/NEJM197902153000701. [DOI] [PubMed] [Google Scholar]
- 13.Farrell LN, Strauss JS, Stranieri AM. The treatment of severe cystic acne with 13-cis-retinoic acid: Evaluation of sebum production and the clinical response in a multiple-dose trial. J Am Acad Dermatol. 1980;3:602–11. doi: 10.1016/s0190-9622(80)80074-0. [DOI] [PubMed] [Google Scholar]
- 14.Goldstein JA, Socha-Szott A, Thomsen RJ, Pochi PE, Shalita AR, Strauss JS. Comparative effect of isotretinoin on acne and sebaceous gland secretion. J Am Acad Dermatol. 1982;6(Suppl):760–5. doi: 10.1016/s0190-9622(82)70066-0. [DOI] [PubMed] [Google Scholar]
- 15.Stainforth JM, Layton AM, Taylor JP, Cunliffe WJ. Isotretinoin for the treatment of acne vulgaris: Which factors may predict the need for more than one course? Br J Dermatol. 1993;129:297–301. doi: 10.1111/j.1365-2133.1993.tb11850.x. [DOI] [PubMed] [Google Scholar]
- 16.Leyden JJ. The role of isotretinoin in the treatment of acne: Personal observations. J Am Acad Dermatol. 1998;39:S45–9. doi: 10.1016/s0190-9622(98)70444-x. [DOI] [PubMed] [Google Scholar]
- 17.Sheth R, Poonevala V. Isotretinoin: An Indian experience. Indian J Dermatol Venereol Leprol. 2001;67:180–2. [PubMed] [Google Scholar]
- 18.Sardana K, Garg VK. Efficacy of low-dose isotretinoin in acne vulgaris. Indian J Dermatol Venereol Leprol. 2010;76:7–13. doi: 10.4103/0378-6323.58672. [DOI] [PubMed] [Google Scholar]
- 19.Hermes B, Praetel C, Henz BM. Medium dose isotretinoin for the treatment of acne. J Eur Acad Dermatol Venereol. 1998;11:117–21. [PubMed] [Google Scholar]
- 20.Mandekou-Lefaki I, Delli F, Teknetzis A, Euthimiadou R, Karakatsanis G. Low-dose schema of isotretinoin in acne vulgaris. Int J Clin Pharmacol Res. 2003;23:41–6. [PubMed] [Google Scholar]
- 21.Amichai B, Shemer A, Grunwald MH. Low-dose isotretinoin in the treatment of acne vulgaris. J Am Acad Dermatol. 2006;54:644–6. doi: 10.1016/j.jaad.2005.11.1061. [DOI] [PubMed] [Google Scholar]
- 22.Akman A, Durusoy C, Senturk M, Koc CK, Soyturk D, Alpsoy E. Treatment of acne with intermittent and conventional isotretinoin: A randomized, controlled multicenter study. Arch Dermatol Res. 2007;299:467–73. doi: 10.1007/s00403-007-0777-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Sardana K, Garg VK, Sehgal VN, Mahajan S, Bhushan P. Efficacy of fixed low-dose isotretinoin (20 mg, alternate days) with topical clindamycin gel in moderately severe acne vulgaris. J Eur Acad Dermatol Venereol. 2009;23:556–60. doi: 10.1111/j.1468-3083.2008.03022.x. [DOI] [PubMed] [Google Scholar]
- 24.Agarwal US, Besarwal RK, Bhola K. Oral isotretinoin in different dose regimens for acne vulgaris: A randomized comparative trial. Indian J Dermatol Venereol Leprol. 2011;77:688–94. doi: 10.4103/0378-6323.86482. [DOI] [PubMed] [Google Scholar]
- 25.De D, Kanwar AJ. Combination of low-dose isotretinoin and pulsed oral azithromycin in the management of moderate to severe acne: A preliminary open-label, prospective, non-comparative, single-centre study. Clin Drug Investig. 2011;31:599–604. doi: 10.2165/11539570-000000000-00000. [DOI] [PubMed] [Google Scholar]
- 26.El-Sherif NA, Greiw AS, Benamer AM. Daily Low Dose versus Intermittent Isotretinoin Regimens. Ibnosina J Med BS. 2013;5:296–302. [Google Scholar]
- 27.Rao PK, Bhat RM, Nandakishore B, Dandakeri S, Martis J, Kamath GH. Safety and efficacy of low-dose isotretinoin in the treatment of moderate to severe acne vulgaris. Indian J Dermatol. 2014;59:316. doi: 10.4103/0019-5154.131455. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Borghi A, Mantovani L, Minghetti S, Virgili A, Bettoli V. Acute acne flare following isotretinoin administration: Potential protective role of low starting dose. Dermatology. 2009;218:178–80. doi: 10.1159/000182270. [DOI] [PubMed] [Google Scholar]
- 29.Wysowski DK, Pitts M, Beitz J. An analysis of reports of depression and suicide in patients treated with isotretinoin. J Am Acad Dermatol. 2001;45:515–9. doi: 10.1067/mjd.2001.117730. [DOI] [PubMed] [Google Scholar]
- 30.Hazen PG, Carney JF, Walker AE, Stewart JJ. Depression-a side effect of 13-cis-retinoic acid therapy. J Am Acad Dermatol. 1983;9:278–9. doi: 10.1016/s0190-9622(83)80154-6. [DOI] [PubMed] [Google Scholar]


