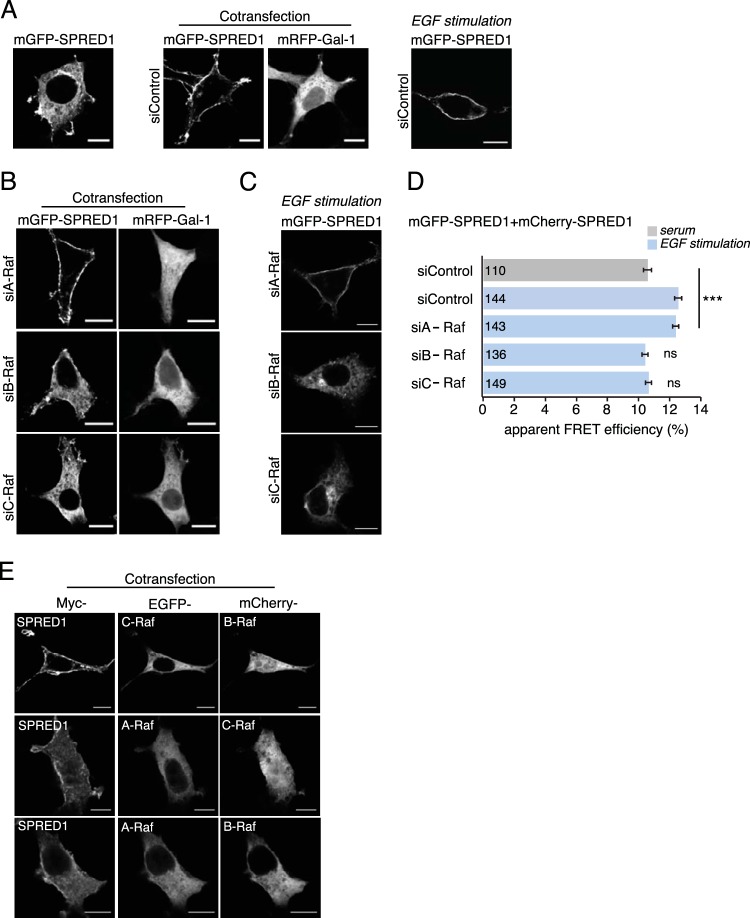
FIG 2.
Silencing of B-Raf or C-Raf abrogates SPRED1 plasma membrane translocation. (A) Gal-1- or EGF-induced SPRED1 localization under nontargeting control siRNA treatment. (B) A-Raf, B-Raf, and C-Raf were first silenced with specific siRNAs (si- prefixes) in HEK cells for 24 h, and then the cells were transfected with mGFP-SPRED1 and with mRFP–Gal-1 for another 24 h. Scale bars, 10 μm. (C) EGF-induced SPRED1 localization after knockdown of individual Raf proteins. The cells were first treated with an siRNA for 24 h and then serum starved for 5 h, followed by transfection of mGFP-SPRED1 for another 24 h under serum-starved conditions. After that, the cells were stimulated with EGF (100 ng/ml) for 10 min and fixed. Scale bars, 10 μm. (D) A SPRED1 membrane translocation FRET assay was conducted in HEK cells transiently coexpressing mGFP-/mCherry-SPRED1. HEK cells were first siRNA treated for 24 h, followed by cotransfections of SPRED1 constructs for another 24 h. The cells were kept under normal serum conditions and fixed (gray bar). Other HEK cells were first treated with siRNA for 24 h, followed by cotransfections of SPRED1 constructs for another 24 h. After that, the cells were starved for 5 h and stimulated with EGF (100 ng/ml) for 10 min and fixed (blue bars). Numbers inside the bars correspond to the total number of cells studied in each case. Error bars indicate the standard errors of the means. ***, P < 0.001; ns, nonsignificant. (E) HEK cells were transfected with an EGFP-tagged A-Raf, B-Raf, or C-Raf construct together with myc-tagged SPRED1 for 24 h, followed by labeling with myc tag antibody (Alexa Fluor 647 conjugate). The cells were imaged by confocal microscopy. Scale bars, 10 μm.