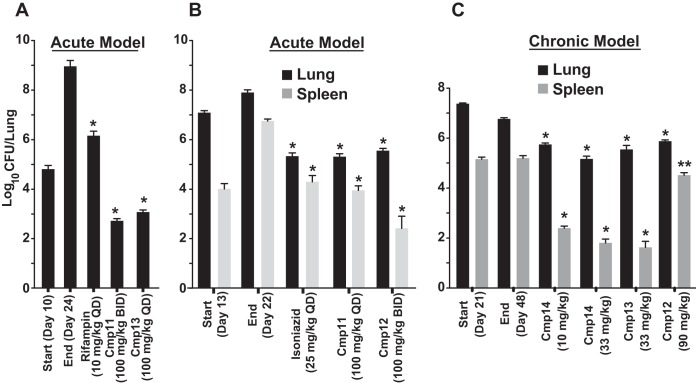
FIG 5.
In vivo efficacy of compounds 11, 12, 13, and 14 in models of acute and chronic of TB infections. (A) In vivo efficacy in a murine GKO (C57BL/6-Ifngtm1ts) model of acute TB. Compounds were dosed orally daily for 14 days after 10 days of infection (start) with a low-dose aerosol of M. tuberculosis Erdman. Mean lung CFU were determined from five mice at the end. (B) In vivo efficacy in a murine GKO (C57BL/6-Ifngtm1ts) model of acute TB. Oral treatment was started 13 days after infection (start) with a low-dose aerosol of M. tuberculosis Erdman lux and continued for 9 consecutive daily treatments until day 21, when mice were sacrificed on day 22 (end). Mean lung CFU were determined from five mice at the end. (C) In vivo efficacy in a murine BALB/c model of chronic TB infection. Compounds were dosed orally 5 days a week for 4 weeks after infection with M. tuberculosis Erdman with a low-dose aerosol 21 days prior (start). Lung and spleen CFU were determined from six mice at the end. **, P < 0.01; *, P < 0.001 (by pairwise multiple-comparison procedures [Tukey test] compared to controls).