Abstract
The phenotypic expression of methicillin resistance among coagulase-negative staphylococci (CoNS) is heterogeneous regardless of the presence of the mecA gene. The potential discordance between phenotypic and genotypic results has led to the use of vancomycin for the treatment of CoNS infective endocarditis (IE) regardless of methicillin MIC values. In this study, we assessed the outcome of methicillin-susceptible CoNS IE among patients treated with antistaphylococcal β-lactams (ASB) versus vancomycin (VAN) in a multicenter cohort study based on data from the International Collaboration on Endocarditis (ICE) Prospective Cohort Study (PCS) and the ICE-Plus databases. The ICE-PCS database contains prospective data on 5,568 patients with IE collected between 2000 and 2006, while the ICE-Plus database contains prospective data on 2,019 patients with IE collected between 2008 and 2012. The primary endpoint was in-hospital mortality. Secondary endpoints were 6-month mortality and survival time. Of the 7,587 patients in the two databases, there were 280 patients with methicillin-susceptible CoNS IE. Detailed treatment and outcome data were available for 180 patients. Eighty-eight patients received ASB, while 36 were treated with VAN. In-hospital mortality (19.3% versus 11.1%; P = 0.27), 6-month mortality (31.6% versus 25.9%; P = 0.58), and survival time after discharge (P = 0.26) did not significantly differ between the two cohorts. Cox regression analysis did not show any significant association between ASB use and the survival time (hazard ratio, 1.7; P = 0.22); this result was not affected by adjustment for confounders. This study provides no evidence for a difference in outcome with the use of VAN versus ASB for methicillin-susceptible CoNS IE.
INTRODUCTION
In addition to being a leading cause of catheter-related bloodstream infection (1), coagulase-negative staphylococci (CoNS) are an important cause of infective endocarditis (IE), accounting for 16% of prosthetic valve endocarditis (PVE) cases and 7.8% native valve endocarditis (NVE) cases (2). Although CoNS are generally considered low-virulence organisms, high rates of valvular abscess formation, congestive heart failure, and mortality are characteristic of CoNS IE (2).
The management of CoNS IE is complicated not only by the high level of methicillin resistance among CoNS strains but also by the heterogeneous expression of methicillin resistance (3). In staphylococci, including CoNS species, methicillin resistance is mediated by the expression of an additional penicillin-binding protein (PBP), designated PBP 2a, leading to resistance to most penicillins, cephalosporins, and carbapenems, except for the recently introduced cephalosporin agents ceftobiprole and ceftaroline. PBP 2a exhibits considerably reduced binding affinities for most β-lactam antibiotics compared to those of the intrinsic set of staphylococcal PBPs found in methicillin-susceptible Staphylococcus aureus (MSSA) and methicillin-susceptible Staphylococcus epidermidis strains (i.e., PBPs 1 to 4) (4–6). PBP 2a is encoded by the mecA gene, which is part of a mobile genetic element designated staphylococcal cassette chromosome mec (7). Conventional antimicrobial susceptibility testing of CoNS is based on the reference methods of the Clinical and Laboratory Standards Institute (CLSI; available at http://www.clsi.org) or of the European Committee on Antimicrobial Susceptibility Testing (EUCAST; available at http://www.eucast.org). Heteroresistance describes the phenomenon in which only a minority of cells of a given isolate with genetically encoded methicillin resistance expresses resistance under in vitro conditions, thus creating a false-susceptible result (8). Heteroresistance in CoNS isolates can reduce the sensitivity and specificity of traditional phenotype-based methods for the detection of methicillin resistance. In the attempt to correct this inappropriate characterization of strains as being susceptible to methicillin, in 1995 the CLSI changed the susceptible breakpoint for CoNS strains from 2 μg/ml to 0.25 μg/ml (9). Despite the new lower breakpoints, false-susceptible results (mecA-positive strains classified as being susceptible by MIC testing) and false-resistant results (mecA-negative strains classified as being resistant by MIC testing) have been documented (10–13). Several factors may be responsible for these discrepancies between phenotypic and genotypic results. The results of phenotypic tests may be influenced by technical factors (e.g., inoculum size, addition of 2% NaCl to broth or agar for dilution) (12). Additionally, mechanisms not mediated by mecA may be responsible for the development of methicillin resistance among CoNS strains. In this regard, Suzuki et al. identified two strains lacking mecA, in spite of their resistance to methicillin. Gel electrophoretic analysis revealed some previously undescribed alterations in the PBP pattern (14). Moreover, the identification of a novel mecA homolog, mecC, in methicillin-resistant Staphylococcus aureus and Staphylococcus saprophyticus isolates lacking the classical mecA gene poses new questions about the genetic determinants of methicillin resistance among CoNS strains (15, 16).
Concern over the discordance between phenotypic and genotypic results has led some clinicians to use vancomycin (VAN) for the treatment of CoNS IE even if the methicillin MIC value falls within the susceptible range (17), exposing patients to the potential adverse effects of vancomycin treatment. The recently identified reversion from methicillin susceptibility to methicillin resistance among mecA-positive MSSA isolates within a patient during antibiotic therapy poses further questions regarding treatment strategies. Similar revertant strains may be found not only among MSSA isolates but also among methicillin-susceptible CoNS isolates (18).
In this observational prospective study, we assessed the influence of the antibiotic regimen (antistaphylococcal β-lactam [ASB] agents versus vancomycin) on the outcome of IE due to methicillin-susceptible CoNS.
MATERIALS AND METHODS
Study design.
This observational study was based on data within the International Collaboration on Endocarditis (ICE) Prospective Cohort Study database and the ICE-Plus database. The ICE Prospective Cohort Study (ICE-PCS) database contains prospective data on 5,568 patients with IE from 64 sites in 28 countries collected between 1 January 2000 and 31 December 2006. The ICE-Plus database contains prospective data on 2,019 patients with IE from 29 sites in 16 countries collected between 1 September 2008 and 31 December 2012. A case report form was developed by the ICE group according to standard definitions (19–22). Data for each patient were collected prospectively by site investigators during the index hospitalization and were then sent to the coordinating center for data entry. Both the ICE-PCS and the ICE-Plus databases are maintained at the Duke Clinical Research Institute, which serves as the coordinating center for the ICE studies, and approvals were obtained from the institutional review boards of the Duke University School of Medicine and the participating ICE sites. A detailed description of the ICE organization and the methodologies for data collection and cataloguing has been provided before (19).
Study population.
Patients were included if they met all of the following criteria: (i) they were 18 years of age or older, (ii) they had a diagnosis of definite IE by the modified Duke criteria (19), (iii) they had monomicrobial IE caused by methicillin-susceptible CoNS, and (iv) they received treatment based on either an antistaphylococcal β-lactam (a penicillinase-resistant penicillin or cefazolin) or vancomycin. Patients treated with antistaphylococcal β-lactams were included in the ASB group, while patients treated with vancomycin were included in the VAN group. Patients simultaneously treated with both ASB and VAN were not included in the study.
Definitions.
Susceptibility to methicillin was defined by MIC testing in accordance with EUCAST and CLSI breakpoints (MIC ≤ 0.25 μg/ml, except for Staphylococcus lugdunensis [MIC ≤ 2 μg/ml]) (available at http://www.clsi.org). The presence and expression of the mecA gene among CoNS strains were not used to define methicillin resistance. Persistent bacteremia was defined as persistence of positive blood cultures after 72 h of organism-specific targeted antibacterial treatment (23). Six-month mortality was defined as the mortality rate at 6 months from the time of hospital admission.
Study objectives.
This study principally aimed to compare in-hospital mortality rates between patients treated with ASB and patients treated with VAN for IE due to methicillin-susceptible CoNS. The secondary objectives of the study included the following: (i) to compare the 6-month mortality rates of the ASB and VAN groups and (ii) to assess the overall survival time among patients in the ASB and VAN groups.
Statistical analysis.
Continuous variables are presented as medians with interquartile ranges (IQRs). Categorical variables are presented as frequencies and percentages of the specified group. Comparisons between groups were made with the Fisher exact test or the Kruskal-Wallis test, as appropriate. A two-sided P value of <0.05 was considered statistically significant. The log-rank test was used to estimate the equality of survival functions (24). To adjust for potential bias, a propensity score that reflected the probability that a patient would receive ASB therapy was generated. Factors associated with the receipt of ASB therapy with a P value of <0.2 in univariate analysis were entered into a logistic regression model (IE type, presence of comorbidities, and site of acquisition). The propensity score was derived from the product of all of the odds ratios in the model (25). For example, a patient with native valve IE (odds ratio, 0.8), at least one comorbidity (odds ratio, 1.7), and the community acquisition of IE (odds ratio, 3.2) would have a propensity score of 0.8 × 1.7 × 3.2 = 4.4. The propensity score was then included as an additional value in the Cox analysis. Statistical analyses were performed using SAS Enterprise Guide, version 5.1, software (SAS Institute, Cary, NC).
RESULTS
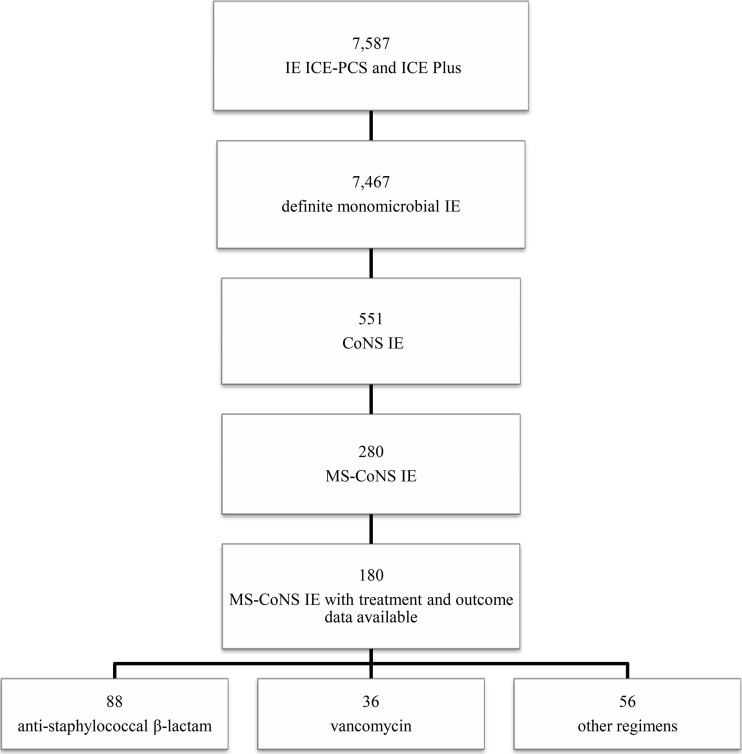
Of the 7,587 patients for whom data were available in the ICE-PCS and ICE-Plus databases, there were 7,467 patients with monomicrobial definite IE. IE was due to methicillin-susceptible CoNS in 280 of these patients, whereas it was due to methicillin-resistant CoNS IE in 271 patients. Detailed treatment and outcome data were available for only 180 patients with methicillin-susceptible CoNS IE. Of these, 88 received an ASB (an antistaphylococcal penicillin in 81 patients and cefazolin in 7 patients) and were included in the ASB group. Thirty-six patients were treated with vancomycin and were included in the VAN group. Patients treated with different antibiotic regimens (i.e., teicoplanin, daptomycin, penicillin, amoxicillin-clavulanate, quinolones) were excluded from the study (n = 56) (Fig. 1). Speciation data were available for 76.6% of the isolates. S. epidermidis and S. lugdunensis were isolated from 75 and 10 patients, respectively. Of the patients with S. lugdunensis IE, 5 were treated with ASB and 5 were treated with VAN. S. capitis, S. haemolyticus, and S. hominis each accounted for 0.02% of the isolates, whereas S. cohnii and S. schleiferi each represented 0.01% of the strains.
FIG 1.
Study population. MS, methicillin susceptible.
Patients in the ASB and VAN groups were from the following geographic regions: for the ASB group, 11.4% were from North America, 2.3% were from South America, 12.5% were from Australia/New Zealand, 65.9% were from Europe, and 8.0% were from Asia; for the VAN group, 34.9% were from North America, 4.7% were from South America, 11.6% were from Australia/New Zealand, and 48.8% were from Europe.
The majority of patients in the study were males (75.0%). The median age was 65.5 years (range 47.0 to 74.0 years). The health care-associated acquisition of CoNS IE was more common among patients treated with VAN than among those treated with an ASB (55.9% versus 25.6%, P < 0.01). Patients in the VAN group had higher rates of a previous episode of IE (14.0% for the VAN group versus 2.3% for the ASB group; P < 0.01) and hemodialysis (20.6% for the VAN group versus 3.9% for the ASB group, P < 0.01). The rate of complications was similar in the two cohorts, except for a higher incidence of stroke among patients treated with VAN (13.9% for the VAN group versus 3.4% for the ASB group, P = 0.04) (Table 1).
TABLE 1.
Baseline characteristics of patients with methicillin-susceptible coagulase-negative staphylococcal infective endocarditis according to treatment (univariate analysis)a
| Characteristic | Result for the following treatment group: |
P valueb | |
|---|---|---|---|
| ASB (n = 88) | VAN (n = 36) | ||
| Median (IQR) age (yr) | 67.5 (47.0–74.5) | 60.5 (46.0–72.0) | 0.29 |
| No. (%) of patients with the following characteristics: | |||
| Male sex | 63/88 (71.6) | 30/36 (83.3) | 0.25 |
| Duration of symptoms of >1 mo before presentation | 20/73 (27.4) | 8/26 (30.8) | 0.74 |
| Presumed type of acquisition | |||
| Community acquired | 61/82 (74.4) | 15/34 (44.1) | <0.01 |
| Health care associated | 21/82 (25.6) | 19/34 (55.9) | |
| Type of IE | |||
| Native valve IE | 54/88 (61.4) | 17/36 (47.2) | |
| Prosthetic valve IE | 16/88 (18.2) | 12/36 (33.3) | 0.17 |
| CEID-related IE | 18/88 (20.5) | 7/36 (19.4) | |
| IE location | |||
| Left-sided IE | 60/72 (83.3) | 30/33 (90.9) | 0.38 |
| Right-sided IE | 11/72 (15.3) | 3/33 (9.1) | 0.54 |
| Left- and right-sided IE | 1/72 (1.4) | 0/33 (0.0) | 1.00 |
| Comorbidities | |||
| Any comorbidity | 23/88 (26.1) | 14/36 (38.9) | 0.16 |
| Dialysis | 3/77 (3.9) | 7/34 (20.6) | <0.01 |
| Diabetes mellitus | 14/87 (16.1) | 7/36 (19.4) | 0.65 |
| Malignancy | 10/88 (11.4) | 2/36 (5.6) | 0.51 |
| Immunosuppressive therapy | 3/88 (3.4) | 2/35 (5.7) | 0.62 |
| Predisposing conditions | |||
| Endocavitary device | 32/88 (36.4) | 11/36 (30.6) | 0.54 |
| History of recent invasive procedure (60 days) | 14/69 (20.3) | 11/33 (33.3) | 0.15 |
| Previous IE episode | 2/88 (2.3) | 5/36 (13.9) | 0.02 |
| Clinical course | |||
| Any complication | 48/88 (54.6) | 22/36 (61.1) | 0.50 |
| Stroke | 3/88 (3.4) | 5/36 (13.9) | 0.04 |
| Systemic embolization other than stroke | 12/88 (13.6) | 6/36 (16.7) | 0.66 |
| New or worsening heart failure | 27/88 (30.7) | 15/36 (41.7) | 0.24 |
| Intracardiac complications (abscess, fistula, perforation) | 21/87 (24.1) | 13/36 (36.1) | 0.18 |
| Paravalvular abscess | 15/87 (17.2) | 10/36 (27.8) | 0.19 |
| Paravalvular fistula | 5/87 (5.8) | 1/36 (2.8) | 0.67 |
| Paravalvular perforation | 7/87 (8.1) | 4/36 (11.1) | 0.73 |
| New conduction abnormality | 8/88 (9.1) | 3/34 (8.8) | 1.00 |
| Persistent bacteremia | 5/88 (5.7) | 3/36 (8.3) | 0.69 |
| Cardiovascular surgery | 57/88 (64.8) | 25/36 (69.4) | 0.62 |
| Outcomes | |||
| In-hospital mortality | 17/88 (19.3) | 4/36 (11.1) | 0.27 |
| 6-mo mortality | 24//76 (31.6) | 7/27 (25.9) | 0.58 |
Only percentages less than 1% are carried to the first decimal place. Abbreviations: IQR, interquartile range; IE, infective endocarditis; CEID, cardiac electronic implantable device.
Statistically significant associations are presented in boldface.
In-hospital mortality did not differ significantly among patients treated with ASB and patients treated with VAN (19.3% versus 11.1%, P = 0.27) (Table 1). However, in-hospital mortality was significantly higher overall among patients with new or worsening heart failure and with intracardiac complications of IE. The duration of symptoms for greater than 1 month and the presence of a cardiac implantable device were associated with lower in-hospital mortality rates (Table 2).
TABLE 2.
In-hospital mortality among patients with methicillin-susceptible CoNS IE (univariate analysis)a
| Characteristic | Result for patients: |
P valueb | |
|---|---|---|---|
| Alive (n = 103) | Dead (n = 21) | ||
| Median (IQR) age (yr) | 66.0 (47.0–73.0) | 63.0 (47.0–74.0) | 0.92 |
| No. (%) of patients with the following characteristics: | |||
| Male sex | 77/103 (74.8) | 16/21 (76.2) | 0.89 |
| Duration of symptoms of >1 mo before presentation | 27/83 (32.5) | 1/16 (6.3) | 0.04 |
| Presumed type of acquisition | |||
| Community acquired | 62/96 (64.6) | 14/20 (70.0) | 0.64 |
| Health care associated | 34/96 (35.4) | 6/20 (30.0) | |
| Type of IE | |||
| Native valve IE | 56/103 (54.4) | 15/21 (71.4) | |
| Prosthetic valve IE | 22/103 (21.4) | 6/21 (28.6) | 0.04 |
| CEID-related IE | 25/103 (24.3) | 0/21 (0.0) | |
| IE location | |||
| Left-sided IE | 70/84 (83.3) | 20/21 (95.2) | 0.29 |
| Right-sided IE | 13/84 (15.5) | 1/21 (4.8) | 0.29 |
| Left- and right-sided IE | 1/84 (1.2) | 0/21 (0.0) | 1.00 |
| Comorbidities | |||
| Any comorbidity | 30/103 (29.1) | 7/21 (33.3) | 0.70 |
| Dialysis | 10/91 (11.0) | 0/20 (0.0) | 0.20 |
| Diabetes mellitus | 17/102 (16.7) | 4/21 (19.1) | 0.76 |
| Malignancy | 8/103 (7.8) | 4/21 (19.1) | 0.12 |
| Immunosuppressive therapy | 3/102 (2.9) | 2/21 (9.5) | 0.20 |
| Predisposing conditions | |||
| Endocavitary device | 39/103 (37.9) | 4/21 (19.1) | 0.10 |
| History of recent invasive procedure (60 days) | 23/85 (27.1) | 2/17 (11.8) | 0.23 |
| Previous IE episode | 7/103 (6.8) | 0/21 (0.0) | 0.60 |
| Clinical course | |||
| Any complication | 51/103 (49.5) | 19/21 (90.5) | <0.01 |
| Stroke | 5/103 (4.9) | 3/21 (14.3) | 0.13 |
| Systemic embolization other than stroke | 13/103 (12.6) | 5/21 (23.8) | 0.19 |
| New or worsening heart failure | 29/103 (28.2) | 13/21 (61.9) | <0.01 |
| Intracardiac complications (abscess, fistula, perforation) | 21/102 (20.6) | 13/21 (61.9) | <0.01 |
| Paravalvular abscess | 17/102 (16.7) | 8/21 (38.1) | 0.04 |
| Paravalvular fistula | 2/102 (2.0) | 4/21 (19.1) | <0.01 |
| Paravalvular perforation | 6/102 (5.9) | 5/21 (23.8) | 0.02 |
| New conduction abnormality | 8/101 (7.9) | 3/21 (14.3) | 0.40 |
| Persistent bacteremia | 8/103 (7.8) | 0/21 (0.0) | 0.35 |
| Cardiovascular surgery | 67/103 (65.1) | 15/21 (71.4) | 0.57 |
| Treatment | |||
| ASB | 71/103 (68.9) | 17/21 (80.9) | 0.27 |
| VAN | 32/103 (31.1) | 4/21 (19.1) | |
Only percentages less than 1% are carried to the first decimal place. Abbreviations: IQR, interquartile range; IE, infective endocarditis; CEID, cardiac electronic implantable device.
Statistically significant associations are presented in boldface.
Similarly, 6-month mortality (data were available for 83% of the study population) did not differ significantly among patients treated with ASB and patients treated with VAN (31.6% versus 25.9%, P = 0.58) (Table 1). The presence of new or worsening heart failure and the development of intracardiac complications were associated with significantly higher 6-month mortality rates (Table 3).
TABLE 3.
Six-month mortality among patients with methicillin-susceptible CoNS IE (univariate analysis)a
| Characteristic | Result for patients: |
P valueb | |
|---|---|---|---|
| Alive (n = 72) | Dead (n = 31) | ||
| Median (IQR) age (yr) | 68.5 (46.5–74.0) | 61.0 (47.0–74.0) | 0.65 |
| No. (%) of patients with the following characteristics: | |||
| Male sex | 55/72 (76.4) | 20/31 (64.5) | 0.21 |
| Duration of symptoms of >1 mo before presentation | 18/55 (32.7) | 4/26 (15.4) | 0.10 |
| Presumed type of acquisition | |||
| Community acquired | 46/66 (69.7) | 20/30 (66.7) | 0.77 |
| Health care associated | 20/66 (30.3) | 10/30 (33.3) | |
| Type of IE | |||
| Native valve IE | 38/72 (52.8) | 20/31 (64.5) | |
| Prosthetic valve IE | 14/72 (19.4) | 8/31 (25.8) | 0.13 |
| CEID-related IE | 20/72 (27.8) | 3/31 (9.7) | |
| IE echocardiographic findings | |||
| Left-sided IE | 46/58 (79.3) | 27/28 (96.4) | 0.06 |
| Right-sided IE | 11/58 (19.0) | 1/28 (3.6) | 0.09 |
| Left- and right-sided IE | 1/58 (1.7) | 0/28 (0.0) | 1.00 |
| Comorbidities | |||
| Any comorbidity | 25/72 (34.7) | 8/31 (25.8) | 0.37 |
| Dialysis | 8/61 (13.1) | 0/29 (0.0) | 0.06 |
| Diabetes mellitus | 16/71 (22.5) | 4/31 (12.9) | 0.26 |
| Malignancy | 6/72 (8.3) | 5/31 (16.1) | 0.30 |
| Immunosuppressive therapy | 3/72 (4.2) | 2/31 (6.5) | 0.64 |
| Predisposing conditions | |||
| Endocavitary device | 31/72 (43.1) | 8/31 (25.8) | 0.10 |
| History of recent invasive procedure (60 days) | 12/59 (20.3) | 4/23 (17.4) | 1.00 |
| Previous IE episode | 3/72 (4.2) | 1/31 (3.2) | 1.00 |
| Clinical course | |||
| Any complication | 33/72 (45.8) | 25/31 (80.7) | <0.01 |
| Stroke | 3/72 (4.2) | 4/31 (12.9) | 0.19 |
| Systemic embolization other than stroke | 10/72 (13.9) | 7/31 (22.6) | 0.28 |
| New or worsening heart failure | 18/72 (25.0) | 16/31 (51.6) | <0.01 |
| Intracardiac complications (abscess, fistula, perforation) | 13/72 (18.1) | 15/31 (48.4) | <0.01 |
| Paravalvular abscess | 11/72 (15.3) | 10/31 (32.3) | 0.04 |
| Paravalvular fistula | 1/72 (1.4) | 4/31 (12.9) | 0.03 |
| Paravalvular perforation | 4/72 (5.6) | 6/31 (19.4) | 0.06 |
| New conduction abnormality | 6/70 (8.6) | 4/31 (12.9) | 0.49 |
| Persistent bacteremia | 6/72 (8.3) | 1/31 (3.2) | 0.67 |
| Cardiovascular surgery | 44/72 (61.1) | 21/31 (67.7) | 0.66 |
| Treatment | |||
| ASB | 52/72 (72.2) | 24/31 (77.4) | 0.58 |
| VAN | 20/72 (27.8) | 7/31 (22.6) | |
Six-month mortality data were available for 83% of the cohort. Only percentages less than 1% are carried to the first decimal place. Abbreviations: IQR, interquartile range; IE, infective endocarditis; CEID, cardiac electronic implantable device.
Statistically significant associations are presented in boldface.
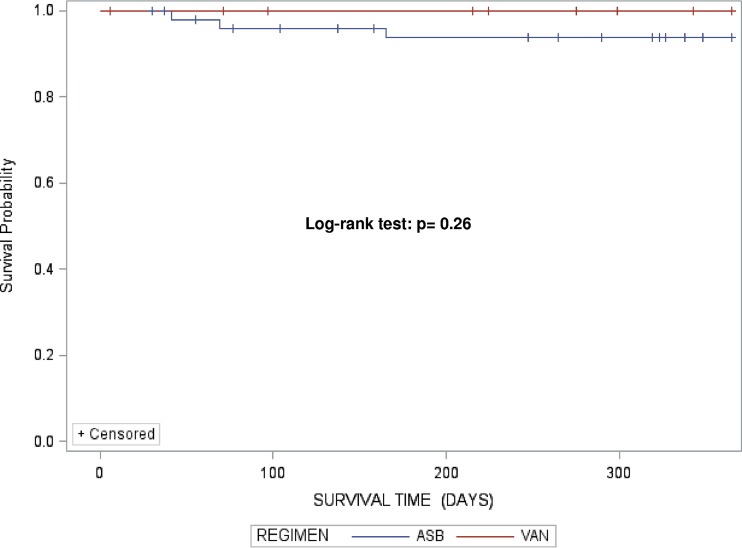
When the overall survival time after discharge was assessed among patients receiving ASB and patients receiving VAN, no significant differences were identified, as shown by the log-rank test (P = 0.26) (Fig. 2). Similarly, Cox regression analysis did not show any significant association between ASB treatment and overall survival time (hazard ratio [HR], 1.7; P = 0.22); this result was not affected by inclusion of the propensity score in the model (HR, 1.7; P = 0.40).
FIG 2.
Product-limit survival estimates.
DISCUSSION
The discordance between phenotypic and genotypic detection of methicillin resistance among CoNS strains has led some experts to recommend VAN use in the setting of all cases of CoNS IE regardless of methicillin MIC values. In order to understand the impact of this strategy on clinical outcomes, we assessed the in-hospital mortality, 6-month mortality, and survival time among methicillin-susceptible CoNS IE patients treated with either ASB or VAN.
This study demonstrated a number of interesting findings. First, there was a substantial clinical use of VAN for the treatment of methicillin-susceptible CoNS IE over a wide geographic area: VAN was used to treat 29.0% of the patients with methicillin-susceptible CoNS IE that fulfilled the study criteria. The use of VAN was also more common in patients with hemodialysis. This observation is not surprising, given the perceived convenience of administering VAN with hemodialysis. However, VAN was also more commonly used in patients with a previous episode of IE and health care-associated IE, which may reflect clinician perceptions regarding the resistance of the CoNS isolate to ASB, despite the MIC result.
Among patients treated with VAN and ASB for methicillin-susceptible CoNS IE, there was no significant difference in the in-hospital mortality, 6-month mortality, and overall survival time.
Although the difference was not statistically significant, there was a trend toward lower rate of in-hospital mortality among patients with methicillin-susceptible CoNS IE treated with VAN; however, this trend did not carry forward to 6-month mortality, the rate of which was similar between groups. Our data suggest that while it is unclear whether antibiotic choice has an impact on short-term outcomes, the long-term outcomes in patients with CoNS infections are likely to be influenced more by other IE-related metrics, such as hemodynamic status (i.e., the presence of congestive heart failure) and valvular mechanical problems (i.e., significant valvular regurgitation). This is consistent with our current understanding of CoNS IE. Although CoNS are indolent pathogens, outcomes tend to be poor because of patient comorbidities and a high rate of heart failure. In our previous study of CoNS IE in the ICE-PCS, chronic illness and congestive heart failure were independently associated with in-hospital mortality (2).
In this study, patients with a longer duration of symptoms prior to presentation had a lower rate of in-hospital mortality. Although this seems counterintuitive, we previously demonstrated that patients with CoNS IE who had symptoms for >1 month prior to presentation were more likely to undergo surgery and had better in-hospital survival (2).
Our investigation has several noteworthy limitations. The small number of patients in this study limited our ability to detect significant differences between groups and to adjust our findings for the presence of confounders. Since this was an observational cohort study, we could not make any definitive inferences between treatment strategies and patient outcomes. Data for this study were derived from sites in the ICE collaboration, which are mostly tertiary care centers with extensive expertise in IE; therefore, the results of this study may be subject to center bias. Methicillin susceptibility was detected only by phenotypic methods, and the presence and the expression of the mecA gene among CoNS strains were not evaluated. Data about the vancomycin blood trough concentration, antimicrobial dosage, and antimicrobial side effects were not collected for inclusion in the database. Finally, combination regimens (including an aminoglycoside and/or rifampin) were used in approximately half of the patients and did not correlate with native versus prosthetic valve status, as is suggested in the current American Heart Association guidelines (26).
In conclusion, our data suggest that patients with methicillin-susceptible CoNS IE treated with ASB rather than VAN do not have significantly different long-term outcomes. Because of the small sample size and the lack of genotypic data, further studies are needed to validate these findings.
ACKNOWLEDGMENTS
Funding for this study was provided by the Instituto de Salud Carlos III and the Ministerio de Economia and Competitividad, Madrid, Spain (Rio Hortega Research Grant CM14/00135 to J.M.P. and intensification research grant INT15/00168 to J.M.M.), and by the American Heart Association (0675027N to V.H.C.). Cubist has provided funding for the ICE microbiology repository.
V.H.C. has received research funding from Merck and writes chapters for UpToDate. None of the other authors has a conflict of interest.
ICE investigators are as follows: in Argentina, Liliana Clara and Marisa Sanchez (Hospital Italiano); José Casabé and Claudia Cortes (Hospital Universitario de la Fundaciòn Favaloro); Francisco Nacinovich, Pablo Fernandez Oses, Ricardo Ronderos, Adriana Sucari, and Jorge Thierer (Instituto Cardiovascular); and Javier Altclas and Silvia Kogan (Sanatorio de la Trinidad Mitre); in Australia, Denis Spelman (Alfred Hospital); Eugene Athan and Owen Harris (Barwon Health); Karina Kennedy and Ren Tan (Canberra Hospital); David Gordon and Lito Papanicolas (Flinders Medical Centre); Tony Korman and Despina Kotsanas (Southern Health); Robyn Dever, Phillip Jones, Pam Konecny, Richard Lawrence, David Rees, and Suzanne Ryan (St. George Hospital); Michael P. Feneley, John Harkness, Phillip Jones, and Suzanne Ryan (St. Vincent's); Phillip Jones and Suzanne Ryan (Sutherland); and Phillip Jones, Jeffrey Post, Porl Reinbott, and Suzanne Ryan (The University of New South Wales); in Austria, Rainer Gattringer and Franz Wiesbauer (Vienna General Hospital); in Brazil, Adriana Ribas Andrade, Ana Cláudia Passos de Brito, and Armenio Costa Guimarães (Ana Neri Hospital); Max Grinberg, Alfredo José Mansur, Rinaldo Focaccia Siciliano, Tania Mara Varejao Strabelli, and Marcelo Luiz Campos Vieira (Heart Institute, University of Sao Paulo Medical School); Regina Aparecida de Medeiros Tranchesi and Marcelo Goulart Paiva (Hospital 9 de Julho); Claudio Querido Fortes (Hospital Universitario Clementino Fraga Filho/UFRJ); Auristela de Oliveira Ramos (Instituto Dante Pazzanese de Cardiologia); Clara Weksler, Giovanna Ferraiuoli, and Wilma Golebiovski (Instituto Nacional de Cardiologia); and Cristiane Lamas (Unigranrio and Instituto Nacional de Cardiologia, Rio de Janeiro, Brazil); in Canada, James A. Karlowsky, Yoav Keynan, Andrew M. Morris, and Ethan Rubinstein (University of Manitoba); in Chile, Sandra Braun Jones and Patricia Garcia (Hospital Clínico, Universidad Católica de Chile) and M. Cereceda, Alberto Fica, and Rodrigo Montagna Mella (Hospital Clinico Universidad de Chile); in Colombia, Ricardo Fernandez, Liliana Franco, Javier Gonzalez, and Astrid Natalia Jaramillo (Clinica Cardiovascular Medellín); in Croatia, Bruno Barsic, Suzana Bukovski, Vladimir Krajinovic, Ana Pangercic, Igor Rudez, and Josip Vincelj (University Hospital for Infectious Diseases); in the Czech Republic, Tomas Freiberger (Ceitec, Masaryk University, Brno, Czech Republic) and Jiri Pol and Barbora Zaloudikova (Centre for Cardiovascular Surgery and Transplantation); in Egypt, Zainab Ashour, Amani El Kholy, Marwa Mishaal, Dina Osama, and Hussien Rizk (Cairo University Medical School); in France, Neijla Aissa, Corentine Alauzet, Francois Alla, Catherine Campagnac, Thanh Doco-Lecompte, and Christine Selton-Suty (CHU Nancy-Brabois); Jean-Paul Casalta, Pierre-Edouard Fournier, Gilbert Habib, Didier Raoult, and Franck Thuny (Faculté de Médecine de Marseille); Francois Delahaye, Armelle Delahaye, and Francois Vandenesch (Hospital Louis Pradel); Erwan Donal, Pierre Yves Donnio, Erwan Flecher, Christian Michelet, Matthieu Revest, and Pierre Tattevin (Pontchaillou University); Florent Chevalier, Antoine Jeu, Jean Paul Rémadi, Dan Rusinaru, and Christophe Tribouilloy (South Hospital Amiens); and Yvette Bernard, Catherine Chirouze, Bruno Hoen, Joel Leroy, and Patrick Plesiat (University Medical Center of Besançon); in Germany, Christoph Naber and Carl Neuerburg (Universitätskliniken Bergmannsheil Bochum) and Bahram Mazaheri, Christoph Naber, and Carl Neuerburg (University Essen); in Greece, Sophia Athanasia, Ioannis Deliolanis, Helen Giamarellou, Tsaganos Thomas, and Efthymia Giannitsioti (Attikon University General Hospital) and Elena Mylona, Olga Paniara, Konstantinos Papanicolaou, John Pyros, and Athanasios Skoutelis (Evangelismos General Hospital of Athens); in India, Gautam Sharma (All India Institute of Medical Sciences) and Johnson Francis, Lathi Nair, Inod Thomas, and Krishnan Venugopal (Medical College Calicut); in Ireland, Margaret M. Hannan and John P. Hurley (Mater Hospitals); in Israel, Amos Cahan, Dan Gilon, Sarah Israel, Maya Korem, and Jacob Strahilevitz (Hadassah-Hebrew University) and Ethan Rubinstein and Jacob Strahilevitz (Tel Aviv University School of Medicine); in Italy, Emanuele Durante-Mangoni, Domenico Iossa, Serena Orlando, Maria Paola Ursi, Pia Clara Pafundi, Fabiana D'Amico, Mariano Bernardo, Susanna Cuccurullo, Giovanni Dialetto, and Riccardo Utili (II Università di Napoli); Marie Françoise Tripodi (University of Salerno); Enrico Cecchi, Francesco De Rosa, Davide Forno, Massimo Imazio, and Rita Trinchero (Maria Vittoria Hospital); Paolo Grossi, Mariangela Lattanzio, and Antonio Toniolo (Ospedale di Circolo Varese); Antonio Goglio, Annibale Raglio, Veronica Ravasio, Marco Rizzi, and Fredy Suter (Ospedali Riuniti di Bergamo); and Giampiero Carosi, Silvia Magri, and Liana Signorini (Spedali Civili, Università di Brescia); in Lebanon, Zeina Kanafani, Souha S. Kanj, and Ahmad Sharif-Yakan (American University of Beirut Medical Center); in Malaysia, Imran Abidin (University of Malaya Medical Center) and Syahidah Syed Tamin (National Heart Institute); in Mexico, Eduardo Rivera Martínez and Gabriel Israel Soto Nieto (Instituto Nacional de Cardiología Ignacio Chávez); in the Netherlands, Jan T. M. van der Meer (University of Amsterdam); in New Zealand, Stephen Chambers and David R. Murdoch (University of Otago); David Holland (Middlemore Hospital); Arthur Morris (Diagnostic Medlab); Nigel Raymond (Wellington Hospital); and Kerry Read (North Shore Hospital); in Romania, Stefan Dragulescu, Adina Ionac, and Cristian Mornos (Victor Babes University of Medicine and Pharmacy); in Russia, O. M. Butkevich (Learning-Scientific Centre of Medical Centre of Russian Presidential Affairs, Government Medical Centre of Russia) and Natalia Chipigina, Ozerecky Kirill, Kulichenko Vadim, and Tatiana Vinogradova (Russian Medical State University); in Saudi Arabia, Jameela Edathodu and Magid Halim (King Faisal Specialist Hospital & Research Center); in Singapore, Yee-Yun Liew and Ru-San Tan (National Heart Centre); in Slovenia, Tatjana Lejko-Zupanc, Mateja Logar, and Manica Mueller-Premru (Medical Center Ljubljana, Slovenia); in South Africa, Patrick Commerford, Anita Commerford, Eduan Deetlefs, Cass Hansa, and Mpiko Ntsekhe (University of Cape Town and Groote Schuur Hospital); in Spain, Manel Almela, Juan Ambrosioni, Manuel Azqueta, Merce Brunet, Pedro Castro, Elisa De Lazzari, Carlos Falces, David Fuster, Guillermina Fita, Cristina Garcia-de-la-Maria, Javier Garcia-Gonzalez, Jose M. Gatell, Jaume Llopis, Francesc Marco, José M. Miró, Asuncion Moreno, José Ortiz, Salvador Ninot, J. Carlos Paré, Juan M. Pericas, Eduard Quintana, Jose Ramirez, Irene Rovira, Elena Sandoval, Marta Sitges, Adrian Tellez, José M. Tolosana, Barbara Vidal, and Jordi Vila (Hospital Clinic—IDIBAPS, University of Barcelona, Barcelona, Spain); Ignasi Anguera, Bernat Font, and Joan Raimon Guma (Hospitál de Sabadell); Javier Bermejo, Emilio Bouza, Miguel Angel Garcia Fernández, Victor Gonzalez-Ramallo, Mercedes Marín, Patricia Muñoz, Miguel Pedromingo, Jorge Roda, Marta Rodríguez-Créixems, and Jorge Solis (Hospital General Universitario Gregorio Marañón); Benito Almirante, Nuria Fernandez-Hidalgo, and Pilar Tornos (Hospital Universitari Vall d'Hebron); and Arístides de Alarcón and Ricardo Parra (Hospital Universitario Virgen del Rocío); in Sweden, Eric Alestig, Magnus Johansson, Lars Olaison, and Ulrika Snygg-Martin (Sahlgrenska Universitetssjukhuset/Östra); in Thailand, Orathai Pachirat, Pimchitra Pachirat, Burabha Pussadhamma, and Vichai Senthong (Khon Kaen University); in the United Kingdom, Anna Casey, Tom Elliott, Peter Lambert, and Richard Watkin (Queen Elizabeth Hospital) and Christina Eyton and John L. Klein (St. Thomas' Hospital); and in the United States, Suzanne Bradley and Carol Kauffman (Ann Arbor VA Medical Center); Roger Bedimo (Dallas VA Medical Center); Vivian H. Chu, G. Ralph Corey, Anna Lisa Crowley, Pamela Douglas, Laura Drew, Vance G. Fowler, Thomas Holland, Tahaniyat Lalani, Daniel Mudrick, Zaniab Samad, Daniel Sexton, Martin Stryjewski, Andrew Wang, and Christopher W. Woods (Duke University Medical Center); Stamatios Lerakis (Emory University); Robert Cantey, Lisa Steed, and Dannah Wray (Medical University of South Carolina); Stuart A. Dickerman (New York University Medical Center); Hector Bonilla, Joseph DiPersio, and Sara-Jane Salstrom (Summa Health System); John Baddley and Mukesh Patel (University of Alabama at Birmingham); Gail Peterson and Amy Stancoven (UT-Southwestern Medical Center); Donald Levine, Jonathan Riddle, and Michael Rybak (Wayne State University); and Christopher H. Cabell (Quintiles). Members of the ICE Coordinating Center are Khaula Baloch, Vivian H. Chu, G. Ralph Corey, Christy C. Dixon, Vance G. Fowler, Jr., Tina Harding, Marian Jones-Richmond, Lawrence P. Park, Bob Sanderford, and Judy Stafford. Members of the ICE Publications Committee are Kevin Anstrom, Eugene Athan, Arnold S. Bayer, Christopher H. Cabell, Vivian H. Chu, G. Ralph Corey, Vance G. Fowler, Jr., Bruno Hoen, A. W. Karchmer, José M. Miró, David R. Murdoch, Daniel J. Sexton, and Andrew Wang. Members of the ICE Steering Committee are Arnold S. Bayer, Christopher H. Cabell, Vivian H. Chu, G. Ralph Corey, David T. Durack, Susannah Eykyn, Vance G. Fowler, Jr., Bruno Hoen, José M. Miró, Phillipe Moreillon, Lars Olaison, Didier Raoult, Ethan Rubinstein, and Daniel J. Sexton.
REFERENCES
- 1.Becker K, Heilmann C, Peters G. 2014. Coagulase-negative staphylococci. Clin Microbiol Rev 4:870–926. doi: 10.1128/CMR.00109-13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Chu VH, Miro JM, Hoen B, Cabell CH, Pappas PA, Jones P, Stryjewski ME, Anguera I, Braun S, Munoz P, Commerford P, Tornos P, Francis J, Oyonarte M, Selton-Suty C, Morris AJ, Habib G, Almirante B, Sexton DJ, Corey GR, Fowler VG Jr. 2009. Coagulase-negative staphylococcal prosthetic valve endocarditis—a contemporary update based on the International Collaboration on Endocarditis: prospective cohort study. Heart 95:570–576. doi: 10.1136/hrt.2008.152975. [DOI] [PubMed] [Google Scholar]
- 3.Dickinson TM, Archer GL. 2000. Phenotypic expression of oxacillin resistance in Staphylococcus epidermidis: roles of mecA transcriptional regulation and resistant-subpopulation selection. Antimicrob Agents Chemother 44:1616–1623. doi: 10.1128/AAC.44.6.1616-1623.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Chambers HF. 1987. Coagulase-negative staphylococci resistant to β-lactam antibiotic in vivo produced penicillin-binding protein 2a. Antimicrob Agents Chemother 31:1919–1924. doi: 10.1128/AAC.31.12.1919. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Chambers HF. 1997. Methicillin resistance in staphylococci: molecular and biochemical basis and clinical implications. Clin Microbiol Rev 10:781–791. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Hartman BJ, Tomasz A. 1984. Low-affinity penicillin-binding protein associated with beta-lactam resistance in Staphylococcus aureus. J Bacteriol 158:513–516. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Katayama Y, Ito T, Hiramatsu K. 2000. A new class of genetic element, staphylococcus cassette chromosome mec, encodes methicillin resistance in Staphylococcus aureus. Antimicrob Agents Chemother 44:1549–1555. doi: 10.1128/AAC.44.6.1549-1555.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Berger-Bachi B. 1994. Expression of resistance to methicillin. Trends Microbiol 2:389–393. doi: 10.1016/0966-842X(94)90617-3. [DOI] [PubMed] [Google Scholar]
- 9.McDonald CL, Maher WE, Fass RJ. 1995. Revised interpretation of oxacillin MICs for Staphylococcus epidermidis based on mecA detection. Antimicrob Agents Chemother 39:982–984. doi: 10.1128/AAC.39.4.982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Frebourg NB, Nouet D, Lemee L, Martin E, Lemeland JF. 1998. Comparison of ATB Staph, Rapid ATB Staph, Vitek, and E-test methods for detection of oxacillin heteroresistance in staphylococci possessing mecA. J Clin Microbiol 36:52–57. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Marshall SA, Wilke WW, Pfaller MA, Jones RN. 1998. Staphylococcus aureus and coagulase-negative staphylococci from blood stream infections: frequency of occurrence, antimicrobial susceptibility, and molecular (mecA) characterization of oxacillin resistance in the SCOPE program. Diagn Microbiol Infect Dis 30:205–214. doi: 10.1016/S0732-8893(97)00212-5. [DOI] [PubMed] [Google Scholar]
- 12.Tenover FC, Jones RN, Swenson JM, Zimmer B, McAllister S, Jorgensen JH. 1999. Methods for improved detection of oxacillin resistance in coagulase-negative staphylococci: results of a multicenter study. J Clin Microbiol 37:4051–4058. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Hussain Z, Stoakes L, Massey V, Diagre D, Fitzgerald V, El Sayed S, Lannigan R. 2000. Correlation of oxacillin MIC with mecA gene carriage in coagulase-negative staphylococci. J Clin Microbiol 38:752–754. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Suzuki E, Hiramatsu K, Yokota T. 1992. Survey of methicillin-resistant clinical strains of coagulase-negative staphylococci for mecA gene distribution. Antimicrob Agents Chemother 36:429–434. doi: 10.1128/AAC.36.2.429. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Kriegeskorte A, Ballhausen B, Idelevich EA, Koch R, Friedrich AW, Karch H, Peters G, Becker K. 2012. Human MRSA isolates with novel genetic homolog, Germany. Emerg Infect Dis 18:1016–1018. doi: 10.3201/eid1806.110910. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Malyszko I, Schwarz S, Hauschild T. 2014. Detection of a new mecC allotype, mecC2, in methicillin-resistant Staphylococcus saprophyticus. J Antimicrob Chemother 69:2003–2005. doi: 10.1093/jac/dku043. [DOI] [PubMed] [Google Scholar]
- 17.Cimolai N, Trombley C, Zaher A. 1997. Oxacillin susceptibility of coagulase-negative staphylococci: role for mecA genotyping and E-test susceptibility testing. Int J Antimicrob Agents 8:121–125. doi: 10.1016/S0924-8579(96)00363-9. [DOI] [PubMed] [Google Scholar]
- 18.Proulx MK, Palace SG, Gandra S, Torres B, Weir S, Stiles T, Ellison RT III, Goguen JD. 2016. Reversion from methicillin susceptibility to methicillin resistance in Staphylococcus aureus during treatment of bacteremia. J Infect Dis 213:1041–1048. doi: 10.1093/infdis/jiv512. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Murdoch DR, Hoen B, Mirò JM, Fowler VG Jr, Bayer AS, Karchmer AW, Pappas PA, Morellion P, Chambers ST, Chu VH, Falcò V, Holland DJ, Jones P, Klein JL, Raymond NJ, Read KM, Tripodi MF, Utili R, Wang A, Woods CW, Cabell CH. 2009. Clinical presentation, etiology, and outcome of infective endocarditis in the 21st century: the International Collaboration on Endocarditis-Prospective Cohort Study. Arch Intern Med 169:463–473. doi: 10.1001/archinternmed.2008.603. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Li JS, Sexton DJ, Mick N, Nettles R, Fowler VG, Ryan T, Bashore T, Corey GR. 2000. Proposed modification to the Duke criteria for the diagnosis of infective endocarditis. Clin Infect Dis 30:633–638. doi: 10.1086/313753. [DOI] [PubMed] [Google Scholar]
- 21.Cabell CH, Abrutyn E. 2002. Progress toward a global understanding of infective endocarditis: early lessons from the International Collaboration on Endocarditis investigation. Infect Dis Clin North Am 16:255–272. doi: 10.1016/S0891-5520(01)00007-1. [DOI] [PubMed] [Google Scholar]
- 22.Wang A, Athan E, Pappas PA, Fowler VG Jr, Olaison L, Parè C, Almirante B, Munoz P, Rizzi M, Naber C, Logar M, Tattevin P, Iarussi DL, Selton-Suty C, Jones SB, Casabè J, Morris A, Corey GR, Cabell CH. 2007. Contemporary clinical profile and outcome of prosthetic valve endocarditis. JAMA 297:1354–1361. doi: 10.1001/jama.297.12.1354. [DOI] [PubMed] [Google Scholar]
- 23.Durack DT, Lukes AS, Bright DK. 1994. New criteria for diagnosis of infective endocarditis. Am J Med 96:200–209. doi: 10.1016/0002-9343(94)90143-0. [DOI] [PubMed] [Google Scholar]
- 24.Leung KM, Elashoff RM, Afifi A. 1997. Censoring issues in survival analysis. Annu Rev Public Health 18:83–104. doi: 10.1146/annurev.publhealth.18.1.83. [DOI] [PubMed] [Google Scholar]
- 25.Newgard C, Hedges JR, Arthur M, Mullins RJ. 2004. Advanced statistics: the propensity score. A method for estimating treatment effect in observational research. Acad Emerg Med 11:953–961. [DOI] [PubMed] [Google Scholar]
- 26.Baddour LM, Wilson WR, Bayer AS, Fowler VG, Tleyjeh IM, Rybak MJ, Barsic B, Lockhart PB, Gewitz MH, Levinson ME, Bolger AF, Steckelberg JM, Baltimore RS, Fink AM, O'Gara P, Taubert KA. 2015. Infective endocarditis in adults: diagnosis, antimicrobial therapy, and management of complications. A scientific statement for healthcare professionals from the American Heart Association. Circulation 132:1435–1486. doi: 10.1161/CIR.0000000000000296. [DOI] [PubMed] [Google Scholar]