Abstract
Aspergillus fumigatus can infect immunocompromised patients, leading to high mortality rates due to the lack of reliable treatment options. This pathogen requires uptake of zinc from host tissues in order to successfully grow and cause virulence. Reducing the availability of that micronutrient could help treat A. fumigatus infections. In this study, we examined the in vitro effects of seven chelators using a bioluminescent strain of A. fumigatus. 1,10-Phenanthroline and N,N,N′,N′-tetrakis(2-pyridylmethyl)ethane-1,2-diamine (TPEN) proved to be the chelators most effective at inhibiting fungal growth. Intraperitoneal administration of either phenanthroline or TPEN resulted in a significant improvement in survival and decrease of weight loss and fungal burden for immunosuppressed mice intranasally infected with A. fumigatus. In vitro both chelators had an indifferent effect when employed in combination with caspofungin. The use of TPEN in combination with caspofungin also significantly increased survival compared to that when using these drugs individually. Our results suggest that zinc chelation may be a valid strategy for dealing with A. fumigatus infections and that both phenanthroline and TPEN could potentially be used either independently or in combination with caspofungin, indicating that their use in combination with other antifungal treatments might also be applicable.
INTRODUCTION
Aspergillus fumigatus is a widespread soil-dwelling fungal saprotroph (1). It is one of the most ubiquitous fungal species with airborne conidia, and it is estimated that all humans inhale several hundred conidia each day (2). These are completely innocuous to immunocompetent hosts. However, the conidia are able to develop and cause invasive pulmonary aspergillosis (IPA) in immunocompromised individuals (3). This disease is difficult to treat, with a mortality rate of 45.6% (4). Commonly used drug treatment options include triazoles such as voriconazole, which inhibit ergosterol synthesis, amphotericin B, which binds to ergosterol and thus results in increased permeability of the cell membrane, and echinocandins such as caspofungin, which inhibit glucan synthesis (5).
Both fungal and bacterial pathogens require cations to grow within their hosts and utilize specialized mechanisms in order to obtain them (6). Zinc is considered essential for all organisms, including pathogens (7). The average concentration of free Zn2+ in human serum is 0.08 μM, which is about 150 times lower than the minimal concentration required for A. fumigatus to grow optimally in a defined liquid medium (8). A. fumigatus possesses three genes, zrfA, zrfB, and zrfC, encoding plasma membrane zinc transporters (8). The zrfA and zrfB genes are required for zinc uptake in acidic Zn-limited environments (7), while zrfC is required for zinc uptake in alkaline environments (9) The zrfC gene is primarily responsible for zinc acquisition within a host's lungs and is required for virulence; zrfA and zrfB contribute to fungal pathogenesis to a lesser extent than zrfC and are not required for virulence if zrfC is present (8). Zinc uptake is induced by the zafA transcriptional activator under Zn-limiting conditions, and its deletion abrogates A. fumigatus virulence (10). Hence, by reducing the availability of zinc, the growth of A. fumigatus might be inhibited, which could have clinical applications as suggested recently (11). In this regard, the nonspecific chelator EDTA has been successfully tested in vitro against Aspergillus (12). In addition, calprotectin, which is a zinc and manganese chelator produced by neutrophils, can inhibit the growth of A. fumigatus in the corneas of immunocompetent mice (13). In contrast, calprotectin was not efficient in inhibiting the fungal growth in lung abscesses of immunosuppressed mice, largely due to the strong zinc-scavenging capacity of ZrfC (8).
The objectives of this study were (i) to test the effects in vivo and in vitro of zinc chelators alone or in combination with antifungal drugs, (ii) to discover whether the chelators are fungicidal or fungistatic, and (iii) to determine the effect of hypoxia and of mammalian sera on the selected chelators.
MATERIALS AND METHODS
The salts solutions used in this study were FeSO4, MgSO4, MnSO4, ZnCl2, and ZnSO4.
The chelators used in this study were the following.
(i) Clioquinol (5-chloro-7-iodo-quinolin-8-ol) is a halogenated 8-hydroxyquinoline (14). It was selected because it has been extensively used as a zinc chelator both in vitro and in vivo (15–19). Clioquinol (Fluca, 33931-100MG-R) was diluted to a 0.05 M stock solution in 100% dimethyl sulfoxide (DMSO). Before use in vitro, the stock solution was further diluted to 5 mM in 100% DMSO and then to 0.5 mM in 10% DMSO.
(ii) Sodium diethyldithiocarbamate trihydrate (DEDTC) is a water-soluble organosulfur compound used as a chelating agent for transition metal ions and to remove zinc from human cell cultures (20, 21). DEDTC (Sigma-Aldrich, D3506-100G) was diluted to a 0.1 M stock solution in distilled water (dH2O).
(iii) Diethylene triamine pentaacetic acid (DTPA) is a membrane-impermeative chelator that has been employed to sequester zinc in cell cultures and is structurally similar to EDTA (22, 23). DTPA (Sigma-Aldrich, D1133-5G) was diluted to a 0.1 M stock solution in 0.5 N HCl.
(iv) Ethylenediamine-N,N′-diacetic acid (EDDA) is an aminopolycarboxylic acid that has been used to chelate zinc in cell cultures (24). EDDA (Sigma-Aldrich, 158186-1G) was diluted to a 0.1 M stock solution in 0.5 N NaOH.
(v) EDTA (calcium disodium salt) is a water-soluble aminopolycarboxylic acid. It was selected because it has been used as a zinc chelator for both A. fumigatus (25) and human cells (26). EDTA (Sigma-Aldrich, ED2SC-250G) was diluted to a 0.1 M stock solution in dH2O.
(vi) 1,10-Phenanthroline is a membrane-permeative (27) heterocyclic compound with a strong inhibitory effect on zinc metallopeptidases (28). It has been employed as a zinc chelator in cell cultures (29) as well as in mice (30) and rats (31). Phenanthroline (Sigma-Aldrich, 131377-5G) was diluted to a 0.2 M stock solution in 100% DMSO. Before use in vitro, the stock solution was further diluted to 0.5 mM in 1% DMSO.
(vii) N,N,N′,N′-tetrakis(2-pyridylmethyl)ethane-1,2-diamine (TPEN) is a membrane-permeative zinc chelator that has also been used extensively in that role in vivo (18, 19, 32, 33) and on A. fumigatus (25). TPEN (Life Technologies, T1210) was diluted to a 0.05 M stock solution in 100% DMSO. Before use in vitro, the stock solution was further diluted to 0.5 mM in 1% DMSO.
Preparation of conidial suspensions.
Conidia were produced from A. fumigatus strain 2/7/1, a modified version of the bioluminescent C3 strain containing a codon-optimized version of the Photinus pyralis luciferase gene. This strain behaved and caused virulence similarly to the wild-type strain (34). Cultures were grown for 7 days on 2% malt agar slants and recovered by vortexing with 0.01% aqueous Tween 20 solution. Homogenous conidial suspensions were collected following filtration through a 40-μm-pore-size filter (Falcon) (35).
In vitro studies of chelator growth inhibition by bioluminescence detection.
The in vitro susceptibility of the A. fumigatus 2/7/1 strain in liquid cultures against chelators was determined by seeding 5 × 104 conidia in a 24-well plate. Each well contained 500 μl of RPMI 1640 cell culture medium (Invitrogen 22409-015; Gibco, France) supplemented with 10% fetal calf serum (FCS) (complete RPMI) (34). For experiments with other sera, 10% human, rat, mouse, or rabbit serum was used to ensure that the serum environment specific to each species does not interfere individually with the properties of the chelators. These were added at different concentrations and at the time points indicated for the specific experiments. Plates were incubated for 10 h at 37°C, 5 μl phosphate-buffered saline (PBS) containing 0.16 mg of d-luciferin was then added to each well, and plates were incubated for 10 min prior to luminescence acquisition on an IVIS 100 system (PerkinElmer, Boston, MA) as previously reported (34). Photons were collected for 1 and 3 min on the high-sensitivity setting. Bioluminescence images were analyzed and the light emission (total photons flux per second) from a region of interest (ROI) quantified with Living Image software (version 3.1; PerkinElmer). Plates were incubated for an additional 5 h at 37°C, and luminescence measurements were repeated as described above. Experiments were repeated twice for each concentration, and cultures were made in triplicate (34). The length of hyphae was measured by taking photographs on an EVOS Core microscope (Thermo Fisher Scientific, Waltham, MA) at a magnification of ×20, followed by measuring the lengths of 100 hyphae for each sample using the ImageJ software. The freehand line tool was used to measure from the conidium to the tip of the longest hypha (34). The MIC-0 and MIC-2 were defined as the lowest concentrations of compound tested that were sufficient to result in at least 95% and 50% reductions in bioluminescence or hyphal length, respectively, compared to those of the positive control (36). The results reflect measurements taken after 10 h of incubation for hyphal lengths and 15 h for luminescence. DMSO did not have a significant effect at the concentrations used for the chelators.
The EUCAST microdilution method was used to obtain MIC and minimum effective concentration (MEC) values, where MIC indicates no visible growth and MEC indicates highly stunted growth, as previously described (37) (38). Briefly, 2 × 104 conidia were seeded in a 96-well plate containing 200 μl of RPMI 1640 medium with 2% glucose per well in the presence of a chelator concentration gradient and incubated for 48 h at 37°C. The dilution range for the tested chelators was 0.065 to 32 mg/liter, and fungal growth was determined using an EVOS Core microscope at a magnification of ×20.
To determine the effects of chelators during a limited time period at the start of incubation, the chelators were added at 0 h and then removed by centrifuging the plate to pellet the conidia, and then the wells were washed twice before adding fresh medium and continuing the incubation. The chelators were removed after 5 h and 8 h, and the incubation was continued for 10 h and 7 h, respectively. The effects of chelators at later growth stages were examined by adding them after 5 h (when conidia start to swell) or 8 h (when conidia start to germinate) had passed, and the incubation was continued for 10 h and 7 h, respectively (34).
For growth under hypoxic conditions, plates were placed in an AnaeroJar (Oxoid, AG0025) according to the manufacturer's instructions in order to generate an environment with <0.1% O2.
The interactions between caspofungin and the chelators were ascertained by determining the fractional inhibitory concentration index (FICI) using a checkerboard method (39). Caspofungin was selected as an example of an established antifungal drug with a mode of action blocking the synthesis of β(1,3)-d-glucan of the fungal cell wall, which differs from that of the chelators acting on zinc metabolism tested in this study. The concentrations used in this assay were closer to one another than those in a 2-fold dilution series in order to obtain greater resolution. The MIC-2 (50% inhibition in luminescence) was employed as an endpoint, as caspofungin is cytostatic rather than cytotoxic (40). The FICI was defined as (Ac/Aa) + (Bc/Ba), where Ac and Bc are the MIC-2 values of the chelator and caspofungin in combination, Aa is the MIC-2 of the chelator, and Ba is the MIC-2 of caspofungin. Interactions were classified as synergistic (FICI of ≤0.5), indifferent (FICI of >0.5 but ≤4), or antagonistic (FICI of >4).
Murine infection and in vivo bioluminescence imaging using an IVIS 100 system.
We used our model of invasive pulmonary aspergillosis (34). Male BALB/cJ mice (23 to 28 g, 8 weeks old) supplied by the R. Janvier breeding center (Le Genest Saint-Isle, France) were used in these experiments. Mice were cared for in accordance with Institut Pasteur guidelines, in compliance with European animal welfare regulation. This study was approved by the ethical committee for animal experimentation (Comité d'Éthique en Experimentation Animale [CETEA], project license number 2013-0020). Mice were weighed daily to monitor changes in body weight. At 4 days and 1 day before infection, each mouse received an immunosuppressive regimen by intraperitoneal (i.p.) injection of 200 μl cyclophosphamide (4 mg/ml). The mice remained immunosuppressed for around 7 days, which was sufficient for them to succumb to infection if left untreated. Mice were inoculated intranasally with a dose of 5 × 104 conidia in 25 μl of PBS–0.01%Tween. Following infection, the chelators, caspofungin, or placebo was administered by i.p. injection at the indicated concentrations in a final volume of 100 μl. The placebo consisted of 10% DMSO in saline solution. The initial concentrations tested for the chelators were based on existing information on murine toxicity of the compounds. The 50% lethal dose (LD50) for intraperitoneal injection of phenanthroline in mice is 75 mg/kg (41). Intraperitoneal injection of ≤10 mg/kg TPEN was well tolerated, while ≥30 mg/kg resulted in death (42). We addressed this problem by using a lower dose of each chelator. The experiments were repeated in order to confirm reproducibility. Combining all the experiments, the control group contained 55 mice, the 5-mg/kg/day TPEN group 25 mice, the 10-mg/kg/day phenanthroline group 30 mice, the 10-mg/kg/day caspofungin group 15 mice, the 5-mg/kg/day caspofungin group 15 mice, and the 5-mg/kg/day caspofungin and 5-mg/kg/day TPEN groups 25 mice. Bioluminescence imaging was started 24 h after infection and was continued every other day. Images were acquired using an IVIS 100 system as previously described (43). Experiments lasted for 14 days postinfection, including 10 days of daily treatment followed by 4 days without treatment to monitor weight recovery.
Inflammatory mediator quantification.
The lungs of dead mice were disrupted in saline using the Retsch Mixer Mill 301 homogenizer. Enzyme-linked immunosorbent assay (ELISA) was used to determine interleukin-6 (IL-6) and CXCL1 concentrations in lung supernatants as specified by the manufacturer (DuoSet; R&D Systems).
Statistical analyses.
For the in vitro tests, the luminescence values of the different cultures in the presence of chelators and/or metal ions were compared to those of the control cultures using unpaired t tests with Welch's correction. Levels of significance for hyphal lengths were calculated using the Mann-Whitney test. For the in vivo tests, survival rates were performed by creating Kaplan-Meier plots and then performing log rank tests. All results are expressed as means ± standard errors of the mean (SEM), and comparisons for survival studies were considered significant if the P value was <0.05. Comparisons of body weights and luminescence within the different groups of mice were performed using the Mann-Whitney test. All tests were performed using GraphPad Prism 6 software. All the MIC-2 and MIC-0 values reported were statistically significant, and the P values indicate the level of significance compared to the positive controls.
RESULTS
The chelators clioquinol, phenanthroline, and TPEN are highly effective at inhibiting A. fumigatus growth.
DEDTC, DTPA, EDDA, and EDTA all had MIC-0 and MIC-2 values equal to or greater than 112.5 mg/liter, while clioquinol, phenanthroline, and TPEN all had MIC-0 and MIC-2 values equal to or less than 6 mg/liter (Table 1). Clioquinol, phenanthroline, and TPEN were thus selected for further experiments, as they were the most effective inhibitors among the compounds examined in this study. The MIC or MEC according to the EUCAST microdilution method after 48 h of incubation was 0.125 mg/liter (MIC) for clioquinol, 4 mg/liter (MIC) for phenanthroline, and 8 mg/liter (MIC) and 0.125 mg/liter (MEC) for TPEN. Both MIC and MEC are presented for TPEN because it was able to cause highly stunted growth at levels that were much lower than those causing complete cessation of growth. These values differ from those in Table 1 due to the difference in medium composition and method.
TABLE 1.
Inhibition of luminescence of A. fumigatus grown for 15 h in the presence of different concentrations of selected chelators
| Chelator | MIC-0 (mg/liter) | MIC-2 (mg/liter) |
|---|---|---|
| Clioquinol | 6 | 6 |
| DEDTC | 900 | 337.5 |
| DTPA | 800 | 300 |
| EDDA | >700 | >700 |
| EDTA | >1,500 | 750 |
| Phenanthroline | 2 | 0.5 |
| TPEN | 3 | 3 |
Zinc neutralizes both phenanthroline and TPEN.
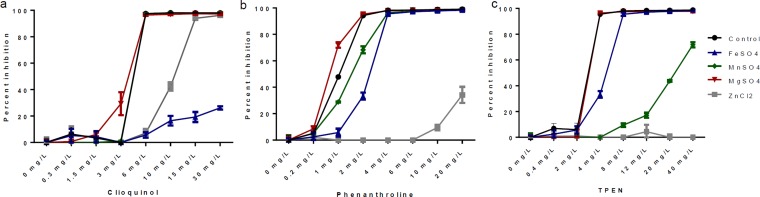
To test the capacity of different metals to overcome the inhibitory effects of clioquinol, phenanthroline, and TPEN on fungal growth, conidia were inoculated in medium containing increasing amounts of these chelators and different salts of metallic ions at a concentration of 1 mM. Fe2+ had a strong effect only on clioquinol and had little effect on phenanthroline and TPEN, suggesting that the latter two chelators did not bind it (Fig. 1). Mg2+had no significant effect on any of the chelators tested, indicating that none of them bound it. Mn2+ had a moderate effect only on TPEN. Zn2+ completely abolished the inhibition of phenanthroline and TPEN but had little effect on clioquinol, indicating that the latter did not sequester Zn under the conditions tested. In summary, clioquinol is strongly inhibited by Fe, phenanthroline is strongly inhibited by Zn, and TPEN is strongly inhibited by Zn and moderately inhibited by Mn.
FIG 1.
Percent inhibition of A. fumigatus grown for 15 h in the presence of 1 mM FeSO4, MgSO4, MnSO4, ZnSO4, or ZnCl2 in addition to clioquinol (a), phenanthroline (b), or TPEN (c).
Chelators have an inhibitory effect that is not growth phase dependent.
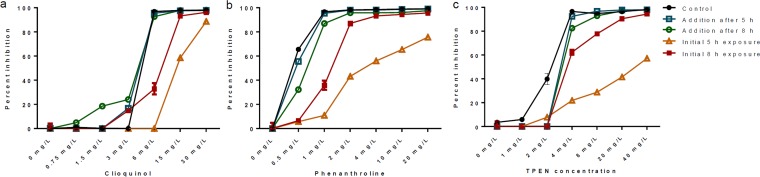
The level of inhibition was time sensitive, as it was reduced if the chelators were left for a shorter period of time (5 h rather than 8 h) in the presence of conidia before being removed (Fig. 2). Clioquinol was faster acting than phenanthroline, which in turn was faster acting than TPEN. The high level of inhibition after chelator removal via washing and replacement of the medium suggests that the chelators were fungicidal after 8 h of incubation. Note, however, that this result relies on fungal inability to resume growth rather than on the determination of a MIC and is therefore only indicative. All of the chelators were able to inhibit growth when added after the conidia had swollen (after 5 h of incubation) or germinated (after 8 h of incubation).
FIG 2.
Percent inhibition of A. fumigatus grown in the presence of clioquinol (a), phenanthroline (b), or TPEN (c) with the chelators either added after 5 or 8 h after the initiation of incubation or removed after an initial incubation for 5 or 8 h in their presence, with a total incubation time of 15 h.
Phenanthroline and TPEN are unaffected by hypoxia, while mammalian sera cause a reduction in clioquinol and TPEN efficacy.
Chelator efficacy was tested under hypoxic conditions and in the presence of mammalian sera in order to simulate the conditions within a host. After 15 h of incubation A. fumigatus grown under hypoxic conditions without chelators had luminescence ranging from 3% to 5% of that for the fungus grown under normoxic conditions (see Fig. S1a in the supplemental material). Both the MIC-2 and MIC-0 of clioquinol underwent a significant reduction (P = 0.0001) under hypoxia compared to normoxia (see Fig. S1b in the supplemental material). No significant differences were observed in the MIC values for the other chelators. These findings indicate that hypoxia did not significantly influence the efficacy of phenanthroline and TPEN, whereas it appeared to decrease that of clioquinol.
A. fumigatus grown in RPMI supplemented with 10% human serum showed luminescence that was one-fourth that of FCS-supplemented RPMI. Rat and mouse sera had 1/10 and 1/20 as much, respectively, while rabbit serum and saline, used as a negative control, had levels of luminescence similar to those for FCS-supplemented RPMI (see Fig. S2a in the supplemental material). Clioquinol was most effective in medium containing saline, followed by human, mouse, rabbit, and rat sera, all of which had similar effects, and was least effective in medium containing fetal calf serum (P < 0.0001) (see Fig. S2b to d in the supplemental material). Phenanthroline was almost equally effective with all sera tested and with saline. TPEN was most effective in medium containing saline (P < 0.0001), followed by human and mouse sera (P < 0.0001), and was least effective in the presence of fetal calf, rabbit, and rat sera.
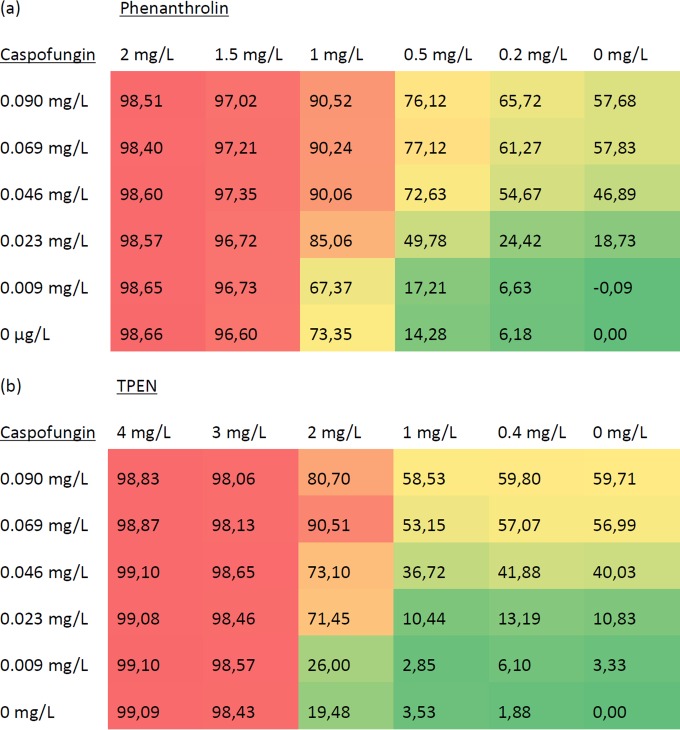
Caspofungin has an indifferent effect with both phenanthroline and TPEN in vitro.
Caspofungin had a MIC-2 of 0.069 mg/liter but no MIC-0, as it was fungistatic rather than fungicidal to Aspergillus and could not cause a 90% reduction in growth (40). Caspofungin and phenanthroline in combination had MIC-2 values of 0.046 mg/liter and 0.5 mg/liter, respectively, while separately these values were 0.069 mg/liter and 1 mg/liter, respectively. Their fractional inhibitory concentration index (FICI) was 1.17. Caspofungin and TPEN in combination had a MIC-2 values of 0.023 mg/liter and 2 mg/liter, respectively, while separately these values were 0.069 mg/liter and 3 mg/liter, respectively. Their FICI was therefore 1.00. The FIC values indicated that both chelators had indifferent effects in combination with caspofungin (Fig. 3).
FIG 3.
Heat maps of percent inhibition of various concentrations of the antifungal caspofungin in combination with various concentrations of the chelator phenanthroline (a) or TPEN (b).
Phenanthroline and TPEN are effective in treating IPA in immunosuppressed mice infected with A. fumigatus.
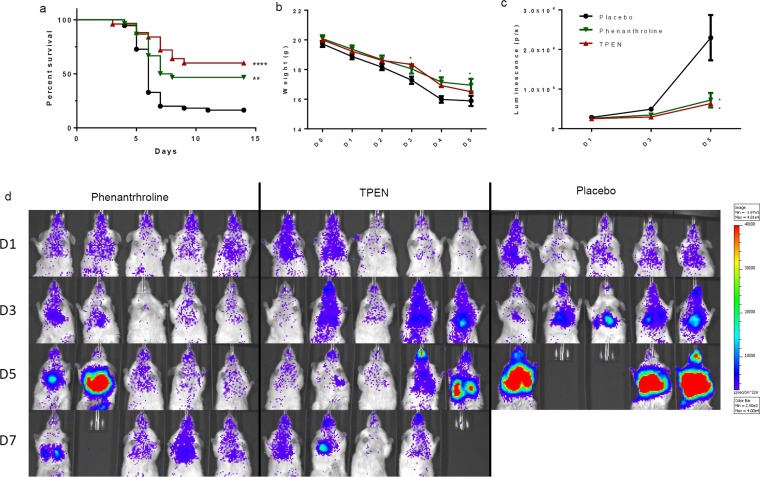
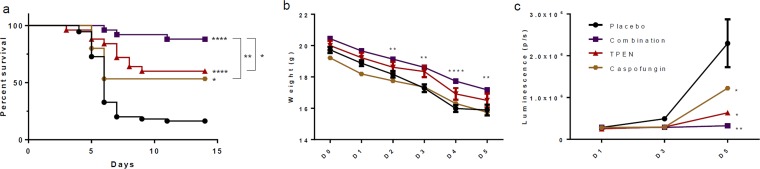
The three most successful chelators in vitro were tested in vivo to determine their effectiveness when used to treat an infected animal model. Mice in the 10% DMSO control group demonstrated a 16% survival rate, with 9 out of 55 mice still alive at the end of the experiment. Clioquinol was tested on infected mice at concentrations of 30 and 15 mg/kg/day. These treatments either decreased or did not significantly alter mouse survival, and clioquinol was abandoned (see Fig. S3 in the supplemental material). TPEN was tested at concentrations of 10, 7.5, and 5 mg/kg/day, while phenanthroline was tested at concentrations of 30, 15, and 10 mg/kg/day. The best effects were observed at concentrations of 5 mg/kg/day for TPEN and 10 mg/kg/day for phenanthroline. Mice treated with 5 mg/kg/day TPEN demonstrated 60% survival, with 15 out of 25 mice alive at the end of the experiment, while those treated with 10 mg/kg/day phenanthroline had 47% survival, with 14 out of 30 mice alive at the end of the experiment (Fig. 4a). Both the phenanthroline and TPEN groups showed weight loss on days 3 and 4 that was significantly lower than that for the control group (Fig. 4b). The TPEN group demonstrated significantly less luminescence than the control group on days 3 and 5, while the phenanthroline group had significantly less luminescence on day 5 (Fig. 4c and d). In summary, both TPEN and phenanthroline significantly increased survival of mice suffering from pulmonary aspergillosis.
FIG 4.
In vivo comparison of effects of treatments on mouse survival using a 10% DMSO placebo, 5 mg/kg/day TPEN, and 10 mg/kg/day phenanthroline. (a) Survival curves. Phenanthroline versus control. P = 0.0023; TPEN versus control, P < 0.0001. (b) Weight averages. Day 3, phenanthroline versus control, P = 0.0116; TPEN versus control, P = 0.0489. Day 4, phenanthroline versus control, P = 0.0333; TPEN versus control, P = 0.0024. (c) Luminescence averages. Day 3, TPEN versus control, P = 0.0122. Day 5, phenanthroline versus control. P = 0.0169; TPEN versus control: P = 0.0140. (d) Examples showing luminescence of mice treated with 5 mg/kg/day TPEN or 10 mg/kg/day phenanthroline and of a 10% DMSO placebo group. As indicated in the scale bar (2.5 × 10E5 to 4 × 10E5 total photons/s), mice with low levels of luminescence (blue) did not develop an infection and survived. Mice with high levels of luminescence (red) developed pulmonary aspergillosis and succumbed to it.
TPEN and caspofungin have an indifferent effect in vivo.
To analyze the effect of TPEN plus caspofungin in vivo, we first determined the optimal concentration of caspofungin for combination treatment and found that 10 mg/kg/day (15 mice) resulted in 100% survival, 7.5 mg/kg/day (10 mice) in 90% survival, 5 mg/kg/day (15 mice) in 53% survival, and 2.5 mg/kg/day (5 mice) in 20% survival. All groups except that receiving the lowest caspofungin concentration had significantly higher survival than the control group (see Fig. S4 in the supplemental material). A combination of 5 mg/kg/day caspofungin and 5 mg/kg/day TPEN gave 88% survival, with 22 out of 25 mice alive at the end of the experiment. In addition, the survival outcome for the combined treatment (caspofungin plus TPEN) turned out to be significantly higher than that for either caspofungin or TPEN alone (Fig. 5a). The combination therapy also significantly reduced the weight loss compared to that for the control group on days 2 to 5. In addition, it significantly reduced the weight loss compared to that for caspofungin monotherapy (Fig. 5b). Finally, the combination therapy and the TPEN treatment allowed a very large reduction in the levels of luminescence compared to those for the control and caspofungin (Fig. 5c). In summary, the combination of TPEN and caspofungin significantly improved mouse survival compared to that using either compound individually.
FIG 5.
In vivo comparison of effects treatments on mouse survival using a 10% DMSO placebo, 5 mg/kg/day TPEN, 5 mg/kg/day caspofungin, and a combination of TPEN and caspofungin. (a) Survival curves. Combination versus control, P < 0.0001; combination versus caspofungin, P = 0.0084; combination versus TPEN, P = 0.0223. (b) Weight averages. Day 2, combination versus placebo, P = 0.0057. Day 3, combination versus placebo, P = 0.0057; TPEN versus placebo, P = 0.0116; combination versus caspofungin, P = 0.0119. Day 4, combination versus placebo, P < 0.0001; TPEN versus placebo, P = 0.0333; combination versus caspofungin, P = 0.0439. Day 5, combination versus placebo, P = 0.0048; combination versus caspofungin, P = 0.0218. (c) Luminescence. Day 3, combination versus placebo, P = 0.0070; TPEN versus placebo, P = 0.0122; caspofungin versus placebo, P = 0.0130. Day 5, combination versus placebo, P = 0.0067; TPEN versus placebo, P = 0.0140. Results are averages for the 10% DMSO placebo group and the groups treated with 5 mg/kg/day caspofungin, the combination of 5 mg/kg/day caspofungin and 5 mg/kg/day TPEN, and 5 mg/kg/day TPEN.
Infection causes exacerbated inflammation patterns in the lungs.
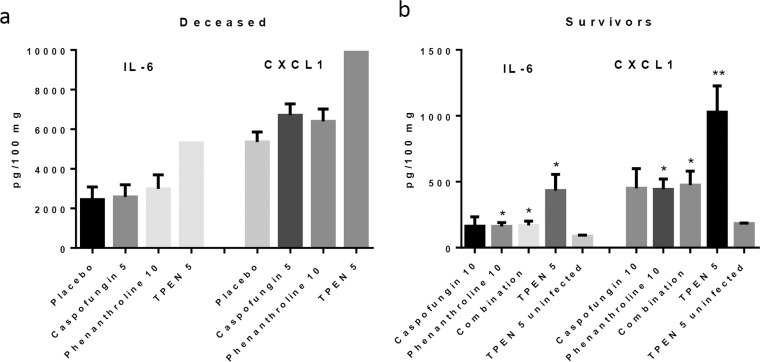
The effects of infection and treatment on the levels of two inflammatory cytokines (IL-6 and CXCL1) within the lungs were examined using ELISA on either mice that died between days 4 and 5 (Fig. 6a) or mice that survived the duration of the experiment and were euthanized on day 14 (Fig. 6b). All the mice that died on days 4 and 5 demonstrated high levels of inflammation, with levels of IL-6 between 2.400 and 5,000 pg/100 mg and those of CXCL1 between 5,000 and 10,000 pg/100 mg. There was no significant difference between treated and untreated mice. The surviving mice all had relatively low levels of cytokines, though the mice treated with chelators or with a combination of a chelator and caspofungin had cytokine levels significantly higher than those in the uninfected mice that received TPEN.
FIG 6.
Levels of the inflammatory cytokines Il-6 and CXCL1 in untreated and treated mice that died from pulmonary aspergillosis infection (a) and treated mice that survived the infection (b). The deceased mouse groups included 8 mice receiving the placebo, 5 receiving caspofungin (5 mg/kg/day), 5 receiving phenanthroline (10 mg/kg/day), and 1 receiving TPEN (5 mg/kg/day). The survivor group included 4 uninfected mice that received TPEN (5 mg/kg/day), 6 that received caspofungin (10 mg/kg/day), 7 that received phenanthroline (10 mg/kg/day), 7 that received the combination (5 mg/kg/day TPEN and 5 mg/kg/day caspofungin), and 10 that received TPEN (5 mg/kg/day). No significant differences were observed among the deceased mice. Phenanthroline versus uninfected, P = 0.0468 for IL-6 and P = 0.0169 for CXCL1; combination versus uninfected, P = 0.0424 for IL-6 and P = 0.0338 for CXCL1; TPEN versus uninfected, P = 0.0204 for IL-6 and P = 0.0022 for CXCL1.
DISCUSSION
We analyzed the effect of several Zn-targeting chelators on fungal growth in vitro prior to testing their efficacy in vivo using a murine model of IPA. Our in vitro assays demonstrated that clioquinol, phenanthroline, and TPEN were the most effective independently of fungal growth stage, and they were selected for use in vivo. They all produced complete inhibition of fungal growth at low concentrations and have been previously used in animal models (18, 30, 33).
In our study, we have shown that phenanthroline and TPEN bound specifically to zinc and not to iron. Their effects are neutralized by zinc. It has been shown that phenanthroline is able to prevent growth of the filamentous fungus Phialophora verrucosa by inhibiting a zinc-dependent metallopeptidase and interfering with fungal morphogenesis (44). In addition, previous research demonstrated that TPEN decreases available zinc but not iron in vitro (45, 46). Moreover, the cytotoxic effect of TPEN is thought to be due to the chelation of intracellular zinc, which interferes with the functioning of essential metalloproteins (47). This is supported by the fact that TPEN strongly promotes the expression of zinc transporter proteins in the fungal pathogen Cryptococcus gattii (48) and the yeast Paracoccidioides brasiliensis (49). Furthermore, an Acinetobacter baumannii strain with a knocked-out zinc transporter is more sensitive to TPEN than the wild type (50).
Testing whether the efficacy of the chelators is affected by hypoxia was important to perform in order to more closely simulate their effects in vivo. It has been shown that inflammation of the lung causes extensive tissue destruction that leads to hypoxic conditions (51, 52). In addition, hypoxia can alter the efficacy of antifungal drugs on Aspergillus, causing an increase in the inhibition by amphotericin B, micafungin, and anidulafungin, a slight decrease in the efficacy of itraconazole, and no effect on voriconazole (53). The effectiveness of azoles is reduced under hypoxic conditions due to the reduction in ergosterol biosynthesis, which is one of the pathways more dependent on partial O2 pressure (pO2). In our study, we found that hypoxia has a strong inhibitory effect on A. fumigatus growth, as previously reported (54). Under hypoxic conditions, the effectiveness of phenanthroline and TPEN remained unchanged, which further increases their potential clinical value, as their activity is unaffected by the low oxygen levels found in hypoxic lung tissue.
Blood serum can affect the growth of different Aspergillus strains to various degrees, as well as the efficacy of different antifungal compounds, either positively or negatively (55); this can vary depending on which species the serum originates from (56). Additionally, there is a correlation between the efficacy of certain antifungals in the presence of serum in vitro and in vivo (57). For these reasons, we decided to compare the effects of the presence or absence of human and other animal sera on A. fumigatus and zinc chelators, as this may provide information regarding their in vivo efficacy. The inhibition of A. fumigatus by human serum agrees with previous findings (55) and is due to the fact that it contains fungal inhibitors (58). The effectiveness of phenanthroline was not affected by any of the sera; however, all tested sera reduced the effectiveness of both clioquinol and TPEN, an effect also observed on other antifungal compounds such as amphotericin B and echinocandins (55). This may be due to binding by albumin or other plasma proteins (59, 60), though there are likely to be other, unknown factors affecting their efficacy as well (57).
Though the serum tests suggested that phenanthroline might be more effective in vivo because it was not inhibited by the components of the serum, TPEN proved to be slightly more effective in treating aspergillosis. It may be that phenanthroline is more readily metabolized by the host and/or that it has a greater difficulty in reaching the lungs.
Our findings demonstrate that zinc chelators can be used to improve survival, to decrease the severity of disease symptoms, and to decrease the fungal burden in the host.
Use of a chelator to sequester a trace metal required for growth by a fungal pathogen has previously been employed against iron. Deferiprone and deferasirox have been successfully used to treat mucormycosis and aspergillosis in mice (61–64) and human patients (65–67). Collectively, these findings and our data indicate that zinc and iron chelators do not interfere with other key metabolic processes of the host and can be a promising treatment option.
Treatment using combinations of different antifungal drugs has been suggested to address the development of resistance among pathogens, as well as to reduce side effects due to drug toxicity to the patient and to achieve antimicrobial synergy (68). Combination treatments are widely used for treating aspergillosis as well as other infections (69). For instance, iron chelators have been used in combination therapy against fungal infections both in laboratory animals (62–64, 70) and in humans (65–67). Combination treatment is thought to improve survival in both patients and animal models (39, 71). Different drug combinations may be synergistic, indifferent, or antagonistic against Aspergillus in vitro (72). The echinocandin antifungal caspofungin (73) was selected as a combination drug because it has favorable outcomes for treating aspergillosis in clinical trials and has been recommended as a treatment option for the disease either as a monotherapy or in combination with other drugs (74). Our in vitro results demonstrated that both phenanthroline and TPEN had indifferent effects when used in combination with caspofungin. This finding agrees with previous findings using EDTA (12) or iron chelators in combination to polyenes (75). The combination treatment with caspofungin and TPEN demonstrated an indifferent effect and significantly improved survival compared to both TPEN and caspofungin monotherapy. Again this result agrees with the finding that an iron chelator in combination with other antifungals was able to improve survival of mice suffering from aspergillosis (64).
The elevated cytokine levels in the lungs of mice that succumbed to pulmonary aspergillosis indicate that these mice died from exacerbated inflammation. This has been observed in chronic granulomatous disease, where patients can succumb to Aspergillus infections from hyperinflammation (76). In our study, the elevated cytokine levels in surviving mice treated with the chelators suggested that the mice had not yet fully returned to normal at 14 days postinfection even though no Aspergillus growth was visible in the lungs using luminescence measurements.
In summary, based on both in vitro and in vivo assays, we conclude that the zinc chelators phenanthroline and TPEN are able to function effectively as antifungal drugs for the treatment of pulmonary aspergillosis in mice either as a monotherapy or as part of a combination therapy. For this reason we are currently undertaking a large-scale in vitro screen of around 60,000 natural and synthetic small molecules in order to identify novel compounds that might interfere with zinc metabolism and may lead to additional treatment options for invasive pulmonary aspergillosis.
Supplementary Material
ACKNOWLEDGMENTS
We are thankful to Catherine Fitting and Véronique Mériaux for their technical assistance with ELISA and EUCAST analyses and to Hervé Waxin from the Centre d'Enseignement and Marie-Anne Nicolas from Plateforme d'Imagerie Dynamique for their assistance with live imaging.
P.L., O.I.-G., H.M.-L., and J.-P.L. were supported by Institut Pasteur PTR 468 funding.
Funding Statement
This research received no specific grant from any funding agency in the public, commercial, or not-for-profit sectors.
Footnotes
Supplemental material for this article may be found at http://dx.doi.org/10.1128/AAC.00324-16.
REFERENCES
- 1.Abad A, Fernandez-Molina JV, Bikandi J, Ramirez A, Margareto J, Sendino J, Hernando FL, Ponton J, Garaizar J, Rementeria A. 2010. What makes Aspergillus fumigatus a successful pathogen? Genes and molecules involved in invasive aspergillosis. Rev Iberoam Micol 27:155–182. doi: 10.1016/j.riam.2010.10.003. [DOI] [PubMed] [Google Scholar]
- 2.Latge JP. 1999. Aspergillus fumigatus and aspergillosis. Clin Microbiol Rev 12:310–350. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Balloy V, Chignard M. 2009. The innate immune response to Aspergillus fumigatus. Microbes Infect 11:919–927. doi: 10.1016/j.micinf.2009.07.002. [DOI] [PubMed] [Google Scholar]
- 4.Steinbach WJ, Marr KA, Anaissie EJ, Azie N, Quan SP, Meier-Kriesche HU, Apewokin S, Horn DL. 2012. Clinical epidemiology of 960 patients with invasive aspergillosis from the PATH Alliance registry. J Infect 65:453–464. doi: 10.1016/j.jinf.2012.08.003. [DOI] [PubMed] [Google Scholar]
- 5.Patterson KC, Strek ME. 2014. Diagnosis and treatment of pulmonary aspergillosis syndromes. Chest 146:1358–1368. doi: 10.1378/chest.14-0917. [DOI] [PubMed] [Google Scholar]
- 6.Schrettl M, Haas H. 2011. Iron homeostasis—Achilles' heel of Aspergillus fumigatus? Curr Opin Microbiol 14:400–405. doi: 10.1016/j.mib.2011.06.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Vicentefranqueira R, Moreno MA, Leal F, Calera JA. 2005. The zrfA and zrfB genes of Aspergillus fumigatus encode the zinc transporter proteins of a zinc uptake system induced in an acid, zinc-depleted environment. Eukaryot Cell 4:837–848. doi: 10.1128/EC.4.5.837-848.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Amich J, Vicentefranqueira R, Mellado E, Ruiz-Carmuega A, Leal F, Calera JA. 2014. The ZrfC alkaline zinc transporter is required for Aspergillus fumigatus virulence and its growth in the presence of the Zn/Mn-chelating protein calprotectin. Cell Microbiol 16:548–564. doi: 10.1111/cmi.12238. [DOI] [PubMed] [Google Scholar]
- 9.Amich J, Vicentefranqueira R, Leal F, Calera JA. 2010. Aspergillus fumigatus survival in alkaline and extreme zinc-limiting environments relies on the induction of a zinc homeostasis system encoded by the zrfC and aspf2 genes. Eukaryot Cell 9:424–437. doi: 10.1128/EC.00348-09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Moreno MA, Ibrahim-Granet O, Vicentefranqueira R, Amich J, Ave P, Leal F, Latge JP, Calera JA. 2007. The regulation of zinc homeostasis by the ZafA transcriptional activator is essential for Aspergillus fumigatus virulence. Mol Microbiol 64:1182–1197. doi: 10.1111/j.1365-2958.2007.05726.x. [DOI] [PubMed] [Google Scholar]
- 11.Vicentefranqueira R, Amich J, Laskaris P, Ibrahim-Granet O, Latge JP, Toledo H, Leal F, Calera JA. 2015. Targeting zinc homeostasis to combat Aspergillus fumigatus infections. Front Microbiol 6:160. doi: 10.3389/fmicb.2015.00160. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Ruhil S, Kumar V, Balhara M, Malik M, Dhankhar S, Kumar M, Kumar Chhillar A. 2014. In vitro evaluation of combination of polyenes with EDTA against Aspergillus spp. by different methods (FICI and CI Model). J Appl Microbiol 117:643–653. doi: 10.1111/jam.12576. [DOI] [PubMed] [Google Scholar]
- 13.Clark HL, Jhingran A, Sun Y, Vareechon C, de Jesus Carrion S, Skaar EP, Chazin WJ, Calera JA, Hohl TM, Pearlman E. 2016. Zinc and manganese chelation by neutrophil S100A8/A9 (calprotectin) limits extracellular Aspergillus fumigatus hyphal growth and corneal infection. J Immunol 196:336–344. doi: 10.4049/jimmunol.1502037. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Mao X, Schimmer AD. 2008. The toxicology of Clioquinol. Toxicol Lett 182:1–6. doi: 10.1016/j.toxlet.2008.08.015. [DOI] [PubMed] [Google Scholar]
- 15.Priel T, Aricha-Tamir B, Sekler I. 2007. Clioquinol attenuates zinc-dependent beta-cell death and the onset of insulitis and hyperglycemia associated with experimental type I diabetes in mice. Eur J Pharmacol 565:232–239. doi: 10.1016/j.ejphar.2007.02.064. [DOI] [PubMed] [Google Scholar]
- 16.Takeda A. 2012. Zinc signaling in the hippocampus and its relation to pathogenesis of depression. J Trace Elem Med Biol 26:80–84. doi: 10.1016/j.jtemb.2012.03.016. [DOI] [PubMed] [Google Scholar]
- 17.Oyama TM, Ishida S, Okano Y, Seo H, Oyama Y. 2012. Clioquinol-induced increase and decrease in the intracellular Zn2+ level in rat thymocytes. Life Sci 91:1216–1220. doi: 10.1016/j.lfs.2012.09.014. [DOI] [PubMed] [Google Scholar]
- 18.Kim JH, Jang BG, Choi BY, Kwon LM, Sohn M, Song HK, Suh SW. 2012. Zinc chelation reduces hippocampal neurogenesis after pilocarpine-induced seizure. PLoS One 7:e48543. doi: 10.1371/journal.pone.0048543. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Takeda A, Iida M, Ando M, Nakamura M, Tamano H, Oku N. 2013. Enhanced susceptibility to spontaneous seizures of noda epileptic rats by loss of synaptic zn(2+). PLoS One 8:e71372. doi: 10.1371/journal.pone.0071372. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Smidt K, Jessen N, Petersen AB, Larsen A, Magnusson N, Jeppesen JB, Stoltenberg M, Culvenor JG, Tsatsanis A, Brock B, Schmitz O, Wogensen L, Bush AI, Rungby J. 2009. SLC30A3 responds to glucose- and zinc variations in beta-cells and is critical for insulin production and in vivo glucose-metabolism during beta-cell stress. PLoS One 4:e5684. doi: 10.1371/journal.pone.0005684. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Siapich SA, Wrubel H, Albanna W, Alnawaiseh M, Hescheler J, Weiergraber M, Luke M, Schneider T. 2010. Effect of ZnCl2 and chelation of zinc ions by N,N-diethyldithiocarbamate (DEDTC) on the ERG b-wave amplitude from the isolated superfused vertebrate retina. Curr Eye Res 35:322–334. doi: 10.3109/02713680903509410. [DOI] [PubMed] [Google Scholar]
- 22.O'Dell BL, Browning JD. 2013. Zinc deficiency induced in Swiss 3T3 cells by a low-zinc medium impairs calcium entry and two mechanisms of entry are involved. Biol Trace Elem Res 152:98–104. doi: 10.1007/s12011-012-9591-6. [DOI] [PubMed] [Google Scholar]
- 23.Cho YE, Lomeda RA, Ryu SH, Lee JH, Beattie JH, Kwun IS. 2007. Cellular Zn depletion by metal ion chelators (TPEN, DTPA and chelex resin) and its application to osteoblastic MC3T3-E1 cells. Nutr Res Pract 1:29–35. doi: 10.4162/nrp.2007.1.1.29. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Krezel A, Maret W. 2006. Zinc-buffering capacity of a eukaryotic cell at physiological pZn. J Biol Inorg Chem 11:1049–1062. doi: 10.1007/s00775-006-0150-5. [DOI] [PubMed] [Google Scholar]
- 25.Lulloff SJ, Hahn BL, Sohnle PG. 2004. Fungal susceptibility to zinc deprivation. J Lab Clin Med 144:208–214. doi: 10.1016/j.lab.2004.07.007. [DOI] [PubMed] [Google Scholar]
- 26.Kim BJ, Kim YH, Kim S, Kim JW, Koh JY, Oh SH, Lee MK, Kim KW, Lee MS. 2000. Zinc as a paracrine effector in pancreatic islet cell death. Diabetes 49:367–372. doi: 10.2337/diabetes.49.3.367. [DOI] [PubMed] [Google Scholar]
- 27.Yang Y, Kawataki T, Fukui K, Koike T. 2007. Cellular Zn2+ chelators cause “dying-back” neurite degeneration associated with energy impairment. J Neurosci Res 85:2844–2855. doi: 10.1002/jnr.21411. [DOI] [PubMed] [Google Scholar]
- 28.Silva BA, Pinto MR, Soares RM, Barreto-Bergter E, Santos AL. 2006. Pseudallescheria boydii releases metallopeptidases capable of cleaving several proteinaceous compounds. Res Microbiol 157:425–432. doi: 10.1016/j.resmic.2005.11.010. [DOI] [PubMed] [Google Scholar]
- 29.Kiss Z. 1994. The zinc chelator 1,10-phenanthroline enhances the stimulatory effects of protein kinase C activators and staurosporine, but not sphingosine and H2O2, on phospholipase D activity in NIH 3T3 fibroblasts. Biochem J 298:93–98. doi: 10.1042/bj2980093. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Chang CH, Yarbro JW, Mann DE Jr, Gautieri RF. 1978. Effects of 1,10-phenanthroline and a zinc complex of 1,10-phenanthroline on nucleic acid synthesis in mouse liver and spleen. J Pharmacol Exp Ther 205:27–32. [PubMed] [Google Scholar]
- 31.Medina C, Videla S, Radomski A, Radomski M, Antolin M, Guarner F, Vilaseca J, Salas A, Malagelada JR. 2001. Therapeutic effect of phenantroline in two rat models of inflammatory bowel disease. Scand J Gastroenterol 36:1314–1319. doi: 10.1080/003655201317097182. [DOI] [PubMed] [Google Scholar]
- 32.Xiao Z, Ehrlich E, Luo K, Xiong Y, Yu XF. 2007. Zinc chelation inhibits HIV Vif activity and liberates antiviral function of the cytidine deaminase APOBEC3G. FASEB J 21:217–222. [DOI] [PubMed] [Google Scholar]
- 33.Cho E, Hwang JJ, Han SH, Chung SJ, Koh JY, Lee JY. 2010. Endogenous zinc mediates apoptotic programmed cell death in the developing brain. Neurotox Res 17:156–166. doi: 10.1007/s12640-009-9085-2. [DOI] [PubMed] [Google Scholar]
- 34.Galiger C, Brock M, Jouvion G, Savers A, Parlato M, Ibrahim-Granet O. 2013. Assessment of efficacy of antifungals against Aspergillus fumigatus: value of real-time bioluminescence imaging. Antimicrob Agents Chemother 57:3046–3059. doi: 10.1128/AAC.01660-12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Lamarre C, Ibrahim-Granet O, Du C, Calderone R, Latge JP. 2007. Characterization of the SKN7 ortholog of Aspergillus fumigatus. Fungal Genet Biol 44:682–690. doi: 10.1016/j.fgb.2007.01.009. [DOI] [PubMed] [Google Scholar]
- 36.Meletiadis J, Mouton JW, Meis JF, Bouman BA, Donnelly PJ, Verweij PE. 2001. Comparison of spectrophotometric and visual readings of NCCLS method and evaluation of a colorimetric method based on reduction of a soluble tetrazolium salt, 2,3-bis [2-methoxy-4-nitro-5-[(sulfenylamino) carbonyl]-2H-tetrazolium-hydroxide], for antifungal susceptibility testing of Aspergillus species. J Clin Microbiol 39:4256–4263. doi: 10.1128/JCM.39.12.4256-4263.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.EUCAST. 2003. Method for the determination of minimum inhibitory concentration (MIC) by broth dilution of fermentative yeasts. Clin Microbiol Infect 9:i–viii. doi: 10.1046/j.1469-0691.2003.00789.x. [DOI] [Google Scholar]
- 38.EUCAST. 2008. EUCAST technical note on the method for the determination of broth dilution minimum inhibitory concentrations of antifungal agents for conidia-forming moulds. Clin Microbiol Infect 14:982–984. doi: 10.1111/j.1469-0691.2008.02086.x. [DOI] [PubMed] [Google Scholar]
- 39.Zhang M, Su X, Sun WK, Chen F, Xu XY, Shi Y. 2014. Efficacy of the combination of voriconazole and caspofungin in experimental pulmonary aspergillosis by different Aspergillus species. Mycopathologia 177:11–18. doi: 10.1007/s11046-013-9719-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Mayr A, Aigner M, Lass-Florl C. 2012. Caspofungin: when and how? The microbiologist's view. Mycoses 55:27–35. doi: 10.1111/j.1439-0507.2011.02039.x. [DOI] [PubMed] [Google Scholar]
- 41.Chemwatch. 2008. 1,10-Phenanthroline material safety data sheet. Santa Cruz Biotechnology, Santa Cruz, CA: http://datasheets.scbt.com/sds/AGHS/EN/sc-255888.pdf. [Google Scholar]
- 42.Adler M, Dinterman RE, Wannemacher RW. 1997. Protection by the heavy metal chelator N,N,N′,N′-tetrakis (2-pyridylmethyl)ethylenediamine (TPEN) against the lethal action of botulinum neurotoxin A and B. Toxicon 35:1089–1100. doi: 10.1016/S0041-0101(96)00215-2. [DOI] [PubMed] [Google Scholar]
- 43.Brock M, Jouvion G, Droin-Bergere S, Dussurget O, Nicola MA, Ibrahim-Granet O. 2008. Bioluminescent Aspergillus fumigatus, a new tool for drug efficiency testing and in vivo monitoring of invasive aspergillosis. Appl Environ Microbiol 74:7023–7035. doi: 10.1128/AEM.01288-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Granato MQ, Massapust Pde A, Rozental S, Alviano CS, dos Santos AL, Kneipp LF. 2015. 1,10-Phenanthroline inhibits the metallopeptidase secreted by Phialophora verrucosa and modulates its growth, morphology and differentiation. Mycopathologia 179:231–242. doi: 10.1007/s11046-014-9832-7. [DOI] [PubMed] [Google Scholar]
- 45.Martin MV, Yates J, Hitchcock CA. 1997. Comparison of voriconazole (UK-109,496) and itraconazole in prevention and treatment of Aspergillus fumigatus endocarditis in guinea pigs. Antimicrob Agents Chemother 41:13–16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Hyun HJ, Sohn JH, Ha DW, Ahn YH, Koh JY, Yoon YH. 2001. Depletion of intracellular zinc and copper with TPEN results in apoptosis of cultured human retinal pigment epithelial cells. Invest Ophthalmol Vis Sci 42:460–465. [PubMed] [Google Scholar]
- 47.Barkalifa R, Hershfinkel M, Friedman JE, Kozak A, Sekler I. 2009. The lipophilic zinc chelator DP-b99 prevents zinc induced neuronal death. Eur J Pharmacol 618:15–21. doi: 10.1016/j.ejphar.2009.07.019. [DOI] [PubMed] [Google Scholar]
- 48.Schneider Rde O, Fogaca Nde S, Kmetzsch L, Schrank A, Vainstein MH, Staats CC. 2012. Zap1 regulates zinc homeostasis and modulates virulence in Cryptococcus gattii. PLoS One 7:e43773. doi: 10.1371/journal.pone.0043773. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Parente AFA, De Rezende TCV, De Castro KP, Bailao AM, Parente JA, Borges CL, Silva LP, Soares CMD. 2013. A proteomic view of the response of Paracoccidioides yeast cells to zinc deprivation. Fungal Biol 117:399–410. doi: 10.1016/j.funbio.2013.04.004. [DOI] [PubMed] [Google Scholar]
- 50.Hood MI, Mortensen BL, Moore JL, Zhang Y, Kehl-Fie TE, Sugitani N, Chazin WJ, Caprioli RM, Skaar EP. 2012. Identification of an Acinetobacter baumannii zinc acquisition system that facilitates resistance to calprotectin-mediated zinc sequestration. PLoS Pathog 8:e1003068. doi: 10.1371/journal.ppat.1003068. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Ibrahim-Granet O, Jouvion G, Hohl TM, Droin-Bergere S, Philippart F, Kim OY, Adib-Conquy M, Schwendener R, Cavaillon JM, Brock M. 2010. In vivo bioluminescence imaging and histopathopathologic analysis reveal distinct roles for resident and recruited immune effector cells in defense against invasive aspergillosis. BMC Microbiol 10:105. doi: 10.1186/1471-2180-10-105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Grahl N, Cramer RA Jr. 2010. Regulation of hypoxia adaptation: an overlooked virulence attribute of pathogenic fungi? Med Mycol 48:1–15. doi: 10.3109/13693780902947342. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Wezensky SJ, Cramer RA Jr. 2011. Implications of hypoxic microenvironments during invasive aspergillosis. Med Mycol 49(Suppl 1):S120–S124. doi: 10.3109/13693786.2010.495139. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Blatzer M, Barker BM, Willger SD, Beckmann N, Blosser SJ, Cornish EJ, Mazurie A, Grahl N, Haas H, Cramer RA. 2011. SREBP coordinates iron and ergosterol homeostasis to mediate triazole drug and hypoxia responses in the human fungal pathogen Aspergillus fumigatus. PLoS Genet 7:e1002374. doi: 10.1371/journal.pgen.1002374. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Elefanti A, Mouton JW, Krompa K, Al-Saigh R, Verweij PE, Zerva L, Meletiadis J. 2013. Inhibitory and fungicidal effects of antifungal drugs against Aspergillus species in the presence of serum. Antimicrob Agents Chemother 57:1625–1631. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Prigitano A, Esposto MC, Tortorano AM. 2014. Comparison of effects of human serum and horse serum on in vitro susceptibility testing of echinocandins. J Chemother 26:62–63. doi: 10.1179/1973947813Y.0000000086. [DOI] [PubMed] [Google Scholar]
- 57.Odabasi Z, Paetznick V, Rex JH, Ostrosky-Zeichner L. 2007. Effects of serum on in vitro susceptibility testing of echinocandins. Antimicrob Agents Chemother 51:4214–4216. doi: 10.1128/AAC.01589-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Even MS, Sandusky CB, Barnard ND. 2006. Serum-free hybridoma culture: ethical, scientific and safety considerations. Trends Biotechnol 24:105–108. doi: 10.1016/j.tibtech.2006.01.001. [DOI] [PubMed] [Google Scholar]
- 59.Ghuman J, Zunszain PA, Petitpas I, Bhattacharya AA, Otagiri M, Curry S. 2005. Structural basis of the drug-binding specificity of human serum albumin. J Mol Biol 353:38–52. doi: 10.1016/j.jmb.2005.07.075. [DOI] [PubMed] [Google Scholar]
- 60.Hobara N, Taketa K. 1976. Electrophoretic studies of clioquinol binding to human serum proteins. Biochem Pharmacol 25:1601–1606. doi: 10.1016/0006-2952(76)90470-6. [DOI] [PubMed] [Google Scholar]
- 61.Ibrahim AS, Edwards JE Jr, Fu Y, Spellberg B. 2006. Deferiprone iron chelation as a novel therapy for experimental mucormycosis. J Antimicrob Chemother 58:1070–1073. doi: 10.1093/jac/dkl350. [DOI] [PubMed] [Google Scholar]
- 62.Ibrahim AS, Gebermariam T, Fu Y, Lin L, Husseiny MI, French SW, Schwartz J, Skory CD, Edwards JE Jr, Spellberg BJ. 2007. The iron chelator deferasirox protects mice from mucormycosis through iron starvation. J Clin Invest 117:2649–2657. doi: 10.1172/JCI32338. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Ibrahim AS, Gebremariam T, French SW, Edwards JE Jr, Spellberg B. 2010. The iron chelator deferasirox enhances liposomal amphotericin B efficacy in treating murine invasive pulmonary aspergillosis. J Antimicrob Chemother 65:289–292. doi: 10.1093/jac/dkp426. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Ibrahim AS, Gebremariam T, Luo G, Fu Y, French SW, Edwards JE Jr, Spellberg B. 2011. Combination therapy of murine mucormycosis or aspergillosis with iron chelation, polyenes, and echinocandins. Antimicrob Agents Chemother 55:1768–1770. doi: 10.1128/AAC.01577-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Reed C, Ibrahim A, Edwards JE Jr, Walot I, Spellberg B. 2006. Deferasirox, an iron-chelating agent, as salvage therapy for rhinocerebral mucormycosis. Antimicrob Agents Chemother 50:3968–3969. doi: 10.1128/AAC.01065-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Busca A, Marmont F, Locatelli F, Limerutti G, Sorrentino MT, Barbui A, Patrono D, Salizzoni M, David E, De Rosa F. 2010. Combined antifungal therapy, iron chelation and surgical resection as treatment of hepatic zygomycosis in a patient with haematological malignancy. Mycoses 53:275–278. doi: 10.1111/j.1439-0507.2009.01708.x. [DOI] [PubMed] [Google Scholar]
- 67.Spellberg B, Andes D, Perez M, Anglim A, Bonilla H, Mathisen GE, Walsh TJ, Ibrahim AS. 2009. Safety and outcomes of open-label deferasirox iron chelation therapy for mucormycosis. Antimicrob Agents Chemother 53:3122–3125. doi: 10.1128/AAC.00361-09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Vitale RG, Afeltra J, Dannaoui E. 2005. Antifungal combinations. Methods Mol Med 118:143–152. [DOI] [PubMed] [Google Scholar]
- 69.Martin-Pena A, Aguilar-Guisado M, Espigado I, Cisneros JM. 2014. Antifungal combination therapy for invasive aspergillosis. Clin Infect Dis 59:1437–1445. doi: 10.1093/cid/ciu581. [DOI] [PubMed] [Google Scholar]
- 70.Zanette RA, Alves SH, Pilotto MB, Weiblen C, Fighera RA, Wolkmer P, Flores MM, Santurio JM. 2013. Iron chelation therapy as a treatment for Pythium insidiosum in an animal model. J Antimicrob Chemother 68:1144–1147. doi: 10.1093/jac/dks534. [DOI] [PubMed] [Google Scholar]
- 71.Panackal AA, Parisini E, Proschan M. 2014. Salvage combination antifungal therapy for acute invasive aspergillosis may improve outcomes: a systematic review and meta-analysis. Int J Infect Dis 28:80–94. doi: 10.1016/j.ijid.2014.07.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Steinbach WJ, Stevens DA. 2003. Review of newer antifungal and immunomodulatory strategies for invasive aspergillosis. Clin Infect Dis 37(Suppl 3):S157–S187. doi: 10.1086/376523. [DOI] [PubMed] [Google Scholar]
- 73.Deresinski SC, Stevens DA. 2003. Caspofungin. Clin Infect Dis 36:1445–1457. doi: 10.1086/375080. [DOI] [PubMed] [Google Scholar]
- 74.Heinz WJ, Einsele H. 2008. Caspofungin for treatment of invasive aspergillus infections. Mycoses 51(Suppl 1):S47–S57. [DOI] [PubMed] [Google Scholar]
- 75.Zarember KA, Cruz AR, Huang CY, Gallin JI. 2009. Antifungal activities of natural and synthetic iron chelators alone and in combination with azole and polyene antibiotics against Aspergillus fumigatus. Antimicrob Agents Chemother 53:2654–2656. doi: 10.1128/AAC.01547-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Romani L, Fallarino F, De Luca A, Montagnoli C, D'Angelo C, Zelante T, Vacca C, Bistoni F, Fioretti MC, Grohmann U, Segal BH, Puccetti P. 2008. Defective tryptophan catabolism underlies inflammation in mouse chronic granulomatous disease. Nature 451:211–215. doi: 10.1038/nature06471. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.