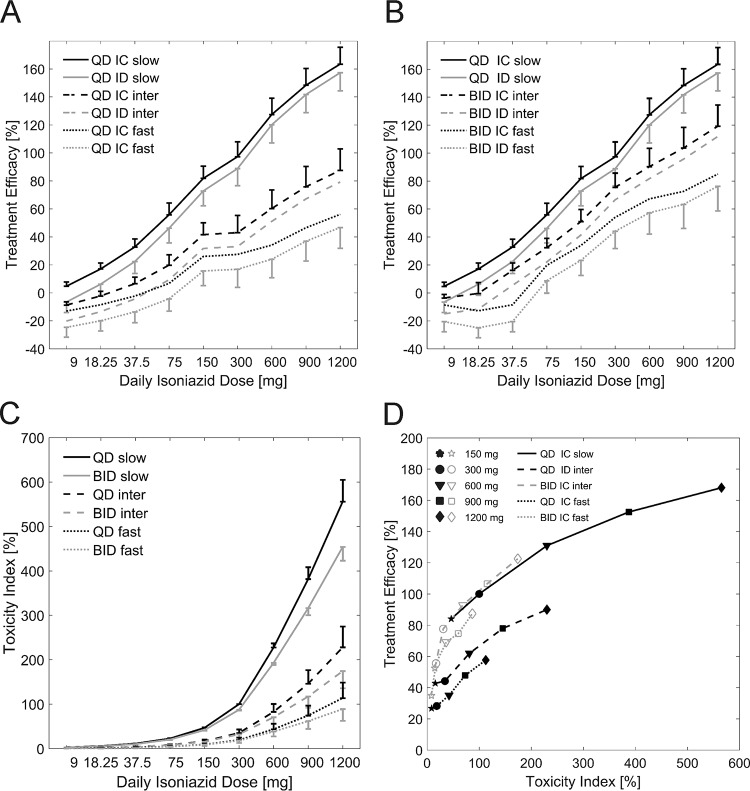
FIG 6.
(A and B) Simulated treatment efficacy for immunocompetent (IC) and immune-deficient (ID) slow, intermediate (inter), and fast NAT2 acetylators receiving INH QD (A) and BID (every 12 h) (B). (C) Simulated toxicity index for slow, intermediate, and fast NAT2 acetylators receiving cumulative QD and BID INH doses ranging from 9 mg to 1,200 mg. Both treatment efficacy and the toxicity index were normalized to those for the slow acetylators receiving 300 mg INH QD. (D) Trade-off between treatment efficacy and toxicity index for INH doses of 1,200 mg, 900 mg, 600 mg, 300 mg, and 150 mg administered QD and BID.