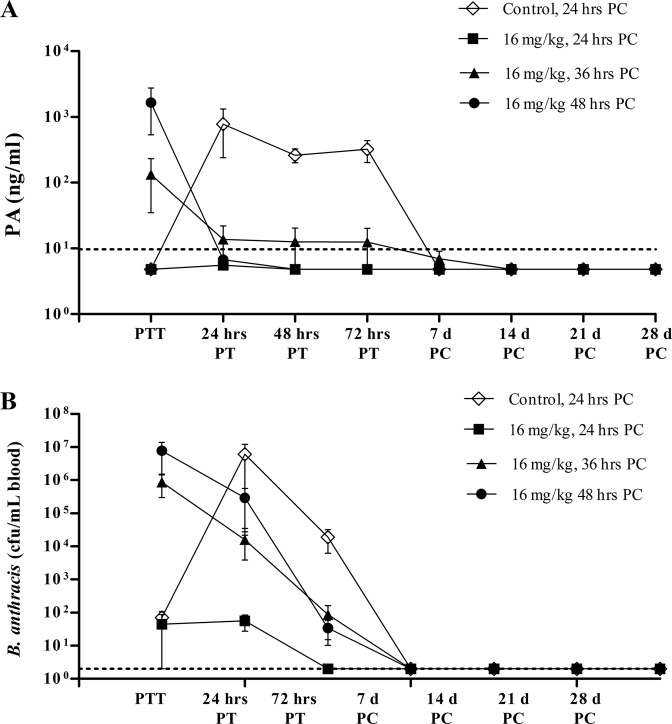
FIG 4.
Development of bacteremia and toxemia during postexposure prophylaxis (PEP). Cynomolgus macaques in study PEP 3 were aerosol challenged with targeted 200 LD50 of B. anthracis spores, and obiltoxaximab was administered at 24, 36, and 48 h postchallenge. Control animals received vehicle at 24 h postchallenge. Peripheral blood samples were collected immediately prior to treatment (PTT) or at indicated times posttreatment (PT) or postchallenge (PC) for the assessment of circulating free PA (A) and quantitative bacteremia (B). Shown are means and standard errors of the means for free PA and bacteremia at each indicated time point. Dotted lines represent LOQ (PA) and LOD (bacteremia). Numbers of animals surviving to each sample collection are indicated at the bottom. For statistical computations, PA levels below the LOQ were replaced with 4.84 ng/ml (1/2 LOQ), and bacteremia levels below the LOD and LOQ were replaced with 2 CFU/ml (1/2 LOD) and 50 CFU/ml (1/2 LOQ), respectively. The control group was comprised of only one survivor starting at day 7.