TABLE 2.
Summary of clinical studies
| Author | N | Study Setting | Type of Surgery | Delirium Criteria | Intervention | Control Group | Intervention Group | Outcomes | ||
|---|---|---|---|---|---|---|---|---|---|---|
|
|
|
|||||||||
| Total | Delirium | Total | Delirium | |||||||
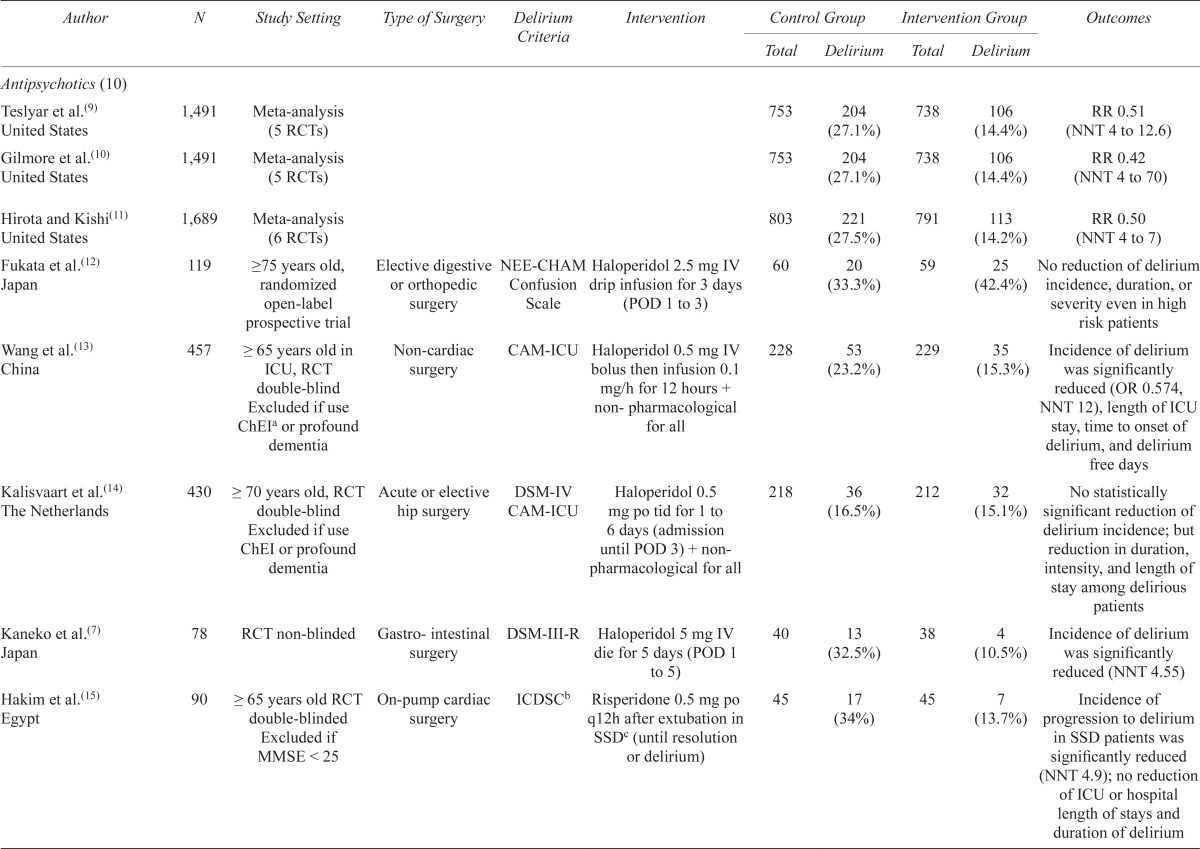
| Antipsychotics (10) | ||||||||||
| Teslyar et al.(9) United States | 1,491 | Meta-analysis (5 RCTs) | 753 | 204 (27.1%) | 738 | 106 (14.4%) | RR 0.51 (NNT 4 to 12.6) | |||
| Gilmore et al.(10) United States | 1,491 | Meta-analysis (5 RCTs) | 753 | 204 (27.1%) | 738 | 106 (14.4%) | RR 0.42 (NNT 4 to 70) | |||
| Hirota and Kishi(11) United States | 1,689 | Meta-analysis (6 RCTs) | 803 | 221 (27.5%) | 791 | 113 (14.2%) | RR 0.50 (NNT 4 to 7) | |||
| Fukata et al.(12) Japan | 119 | ≥75 years old, randomized open-label prospective trial | Elective digestive NEE-CHAM or orthopedic surgery | Confusion Scale | Haloperidol 2.5 mg IV drip infusion for 3 days (POD 1 to 3) | 60 | 20 (33.3%) | 59 | 25 (42.4%) | No reduction of delirium incidence, duration, or severity even in high risk patients |
| Wang et al.(13) China | 457 | ≥ 65 years old in ICU, RCT double-blind Excluded if use ChEIa or profound dementia | Non-cardiac surgery | CAM-ICU | Haloperidol 0.5 mg IV bolus then infusion 0.1 mg/h for 12 hours + non- pharmacological for all | 228 | 53 (23.2%) | 229 | 35 (15.3%) | Incidence of delirium was significantly reduced (OR 0.574, NNT 12), length of ICU stay, time to onset of delirium, and delirium free days |
| Kalisvaart et al.(14) The Netherlands | 430 | ≥ 70 years old, RCT double-blind Excluded if use ChEI or profound dementia | Acute or elective hip surgery | DSM-IV CAM-ICU | Haloperidol 0.5 mg po tid for 1 to 6 days (admission until POD 3) + non pharmacological for all | 218 | 36 (16.5%) | 212 | 32 (15.1%) | No statistically significant reduction of delirium incidence; but reduction in duration, intensity, and length of stay among delirious patients |
| Kaneko et al.(7) Japan | 78 | RCT non-blinded | Gastro- intestinal surgery | DSM-III-R | Haloperidol 5 mg IV die for 5 days (POD 1 to 5) | 40 | 13 (32.5%) | 38 | 4 (10.5%) | Incidence of delirium was significantly reduced (NNT 4.55) |
| Hakim et al.(15) Egypt | 90 | ≥ 65 years old RCT double-blinded Excluded if MMSE < 25 | On-pump cardiac surgery | ICDSCb | Risperidone 0.5 mg po q12h after extubation in SSDc (until resolution or delirium) | 45 | 17 (34%) | 45 | 7 (13.7%) | Incidence of progression to delirium in SSD patients was significantly reduced (NNT 4.9); no reduction of ICU or hospital length of stays and duration of delirium |
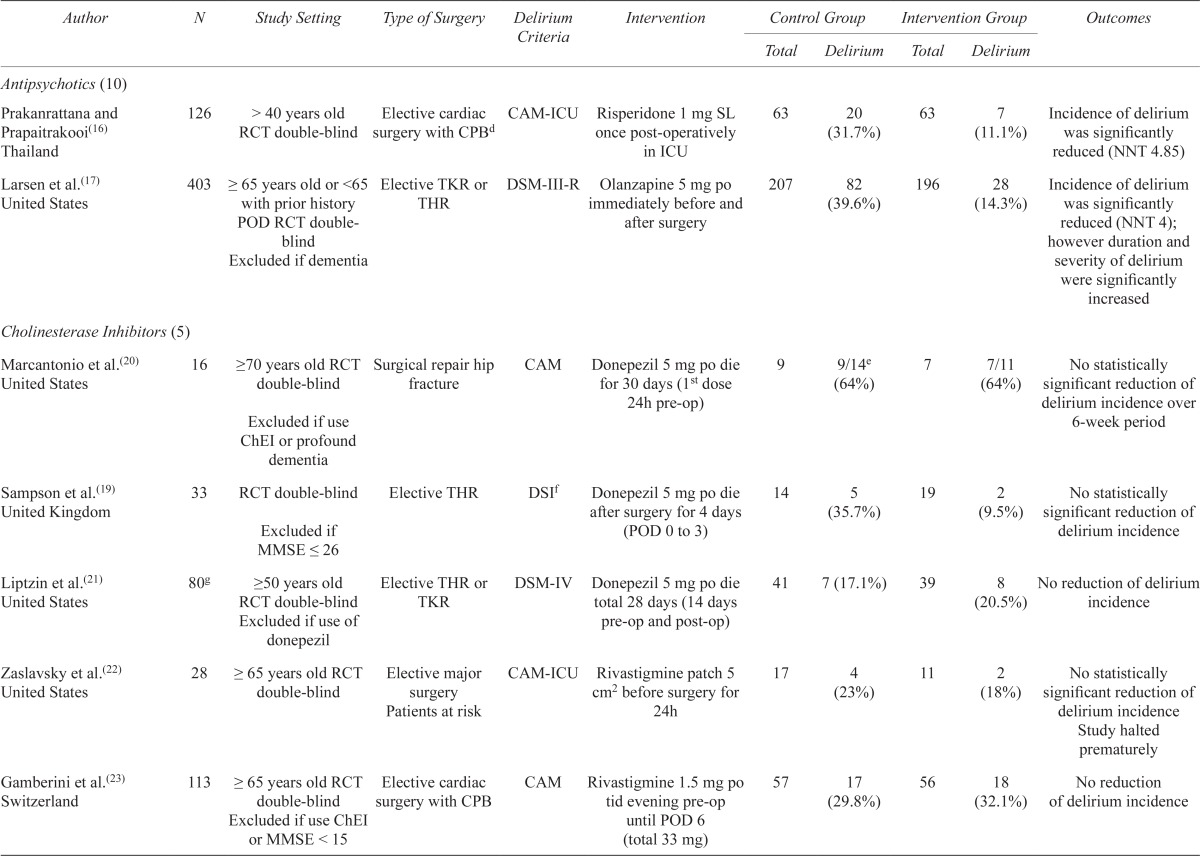
| Prakanrattana and Prapaitrakooi(16) Thailand | 126 | > 40 years old RCT double-blind | Elective cardiac surgery with CPBd | CAM-ICU | Risperidone 1 mg SL once post-operatively in ICU | 63 | 20 (31.7%) | 63 | 7 (11.1%) | Incidence of delirium was significantly reduced (NNT 4.85) |
| Larsen et al.(17) United States | 403 | ≥ 65 years old or <65 with prior history POD RCT double-blind Excluded if dementia |
Elective TKR or THR | DSM-III-R | Olanzapine 5 mg po immediately before and after surgery | 207 | 82 (39.6%) | 196 | 28 (14.3%) | Incidence of delirium was significantly reduced (NNT 4); however duration and severity of delirium were significantly increased |
| Cholinesterase Inhibitors (5) | ||||||||||
| Marcantonio et al.(20) United States | 16 | ≥70 years old RCT double-blind Excluded if use ChEI or profound dementia |
Surgical repair hip fracture | CAM | Donepezil 5 mg po die for 30 days (1st dose 24h pre-op) | 9 | 9/14e (64%) | 7 | 7/11 (64%) | No statistically significant reduction of delirium incidence over 6-week period |
| Sampson et al.(19) United Kingdom | 33 | RCT double-blind Excluded if MMSE ≤ 26 |
Elective THR | DSIf | Donepezil 5 mg po die after surgery for 4 days (POD 0 to 3) | 14 | 5 (35.7%) | 19 | 2 (9.5%) | No statistically significant reduction of delirium incidence |
| Liptzin et al.(21) United States | 80g | ≥50 years old RCT double-blind Excluded if use of donepezil |
Elective THR or TKR | DSM-IV | Donepezil 5 mg po die total 28 days (14 days pre-op and post-op) | 41 | 7 (17.1%) | 39 | 8 (20.5%) | No reduction of delirium incidence |
| Zaslavsky et al.(22) United States | 28 | ≥ 65 years old RCT double-blind | Elective major surgery Patients at risk |
CAM-ICU | Rivastigmine patch 5 cm2 before surgery for 24h | 17 | 4 (23%) | 11 | 2 (18%) | No statistically significant reduction of delirium incidence Study halted prematurely |
| Gamberini et al.(23) Switzerland | 113 | ≥ 65 years old RCT double-blind surgery with CPB Excluded if use ChEI or MMSE < 15 |
Elective cardiac | CAM | Rivastigmine 1.5 mg po tid evening pre-op until POD 6 (total 33 mg) | 57 | 17 (29.8%) | 56 | 18 (32.1%) | No reduction of delirium incidence |
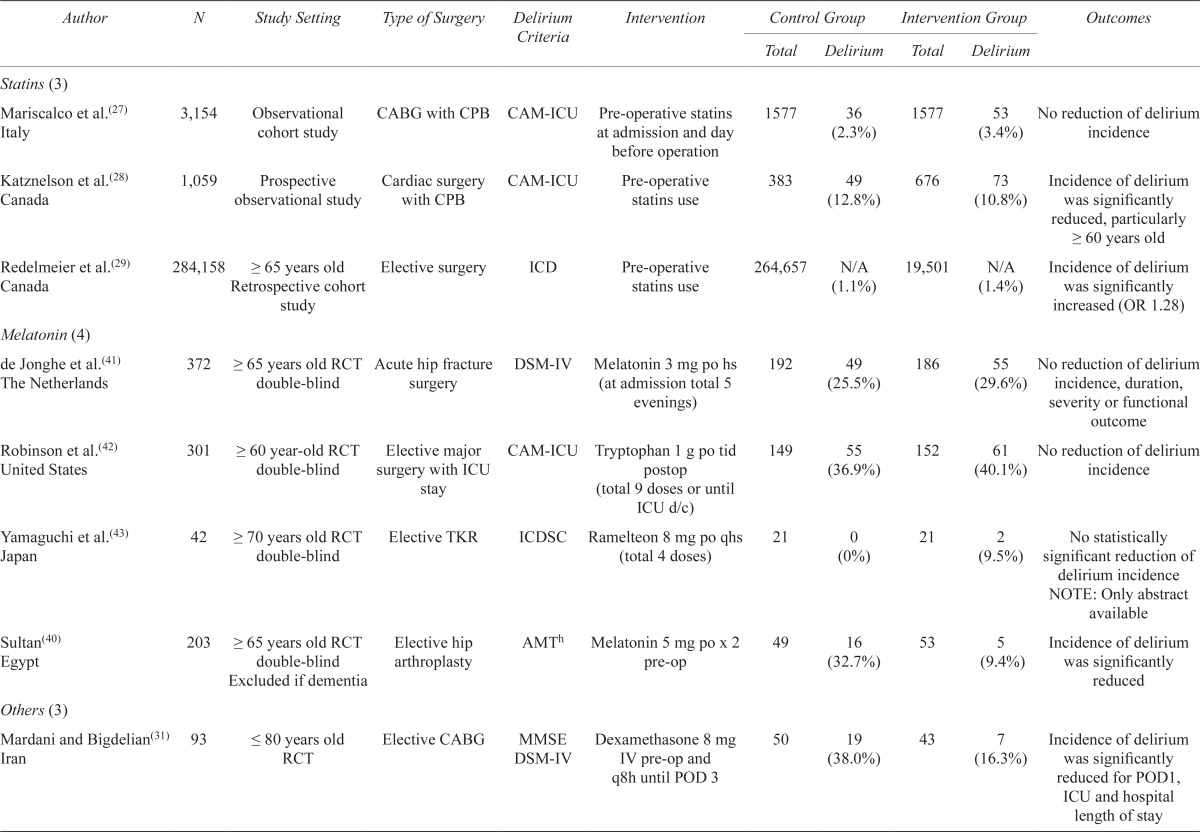
| Statins (3) | ||||||||||
| Mariscalco et al.(27) Italy | 3,154 | Observational cohort study | CABG with CPB | CAM-ICU | Pre-operative statins at admission and day before operation | 1577 | 36 (2.3%) | 1577 | 53 (3.4%) | No reduction of delirium incidence |
| Katznelson et al.(28) Canada | 1,059 | Prospective observational study | Cardiac surgery with CPB | CAM-ICU | Pre-operative statins use | 383 | 49 (12.8%) | 676 | 73 (10.8%) | Incidence of delirium was significantly reduced, particularly ≥ 60 years old |
| Redelmeier et al.(29) Canada | 284,158 | ≥ 65 years old Retrospective cohort study |
Elective surgery | ICD | Pre-operative statins use | 264,657 | N/A (1.1%) | 19,501 | N/A (1.4%) | Incidence of delirium was significantly increased (OR 1.28) |
| Melatonin (4) | ||||||||||
| de Jonghe et al.(41) The Netherlands | 372 | ≥ 65 years old RCT double-blind | Acute hip fracture surgery | DSM-IV | Melatonin 3 mg po hs (at admission total 5 evenings) | 192 | 49 (25.5%) | 186 | 55 (29.6%) | No reduction of delirium incidence, duration, severity or functional outcome |
| Robinson et al.(42) United States | 301 | ≥ 60 year-old RCT double-blind | Elective major surgery with ICU stay | CAM-ICU | Tryptophan 1 g po tid postop (total 9 doses or until ICU d/c) | 149 | 55 (36.9%) | 152 | 61 (40.1%) | No reduction of delirium incidence |
| Yamaguchi et al.(43) Japan | 42 | ≥ 70 years old RCT double-blind | Elective TKR | ICDSC | Ramelteon 8 mg po qhs (total 4 doses) | 21 | 0 (0%) | 21 | 2 (9.5%) | No statistically significant reduction of delirium incidence NOTE: Only abstract available |
| Sultan(40) Egypt | 203 | ≥ 65 years old RCT double-blind Excluded if dementia |
Elective hip arthroplasty | AMTh | Melatonin 5 mg po x 2 pre-op | 49 | 16 (32.7%) | 53 | 5 (9.4%) | Incidence of delirium was significantly reduced |
| Others (3) | ||||||||||
| Mardani and Bigdelian(31) Iran | 93 | ≤ 80 years old RCT | Elective CABG | MMSE DSM-IV | Dexamethasone 8 mg IV pre-op and q8h until POD 3 | 50 | 19 (38.0%) | 43 | 7 (16.3%) | Incidence of delirium was significantly reduced for POD1, ICU and hospital length of stay |
| Leung et al.(36) United States | 21 | ≥ 45 years old RCT double-blind | Spinal surgery | CAM | Gabapentin 900 mg po die pre-op until POD 3 | 12 | 5 (41.7%) | 9 | 0 (0%) | Incidence of delirium was significantly reduced |
| Aizawa et al.(37) Japan | 40 | >70 years old RCT non-double-blind Excluded if ≥ 86 years old or psychiatric disorder |
Gastric or colorectal cancer resection | DSM-IV | DFP (Diazepam IM, Flunitrazepam IV, Pethidine IV) pre-op until POD 3 | 20 | 7 (35.0%) | 20 | 1 (5.0%) | Incidence of delirium was significantly reduced |
Cholinesterase inhibitor.
Intensive Care Delirium Screening Checklist.
Subsyndromal delirium.
Cardiopulmonary bypass.
More than one interview per subject during hospitalization.
Delirium Symptom Interview.
58 patients actually completed the study with 28 days of Donepezil (most stopped after randomization).
Abbreviated Mental Status.