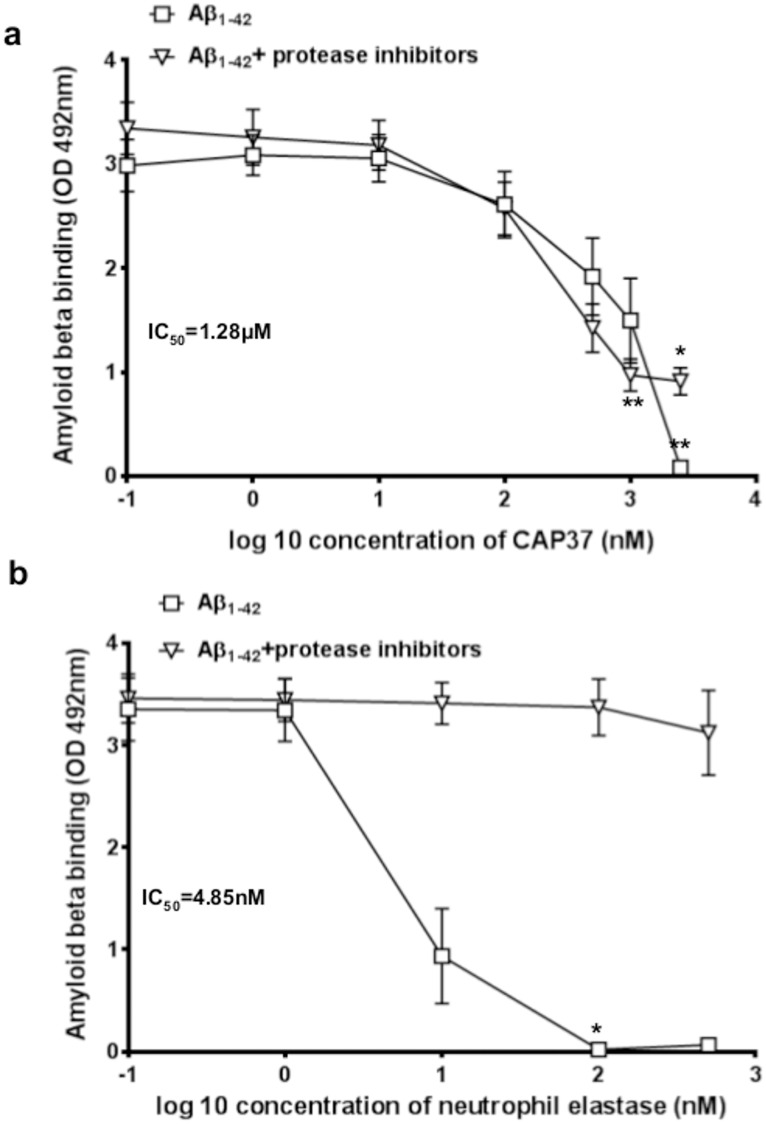
Fig 4. Neutrophil proteins inhibit Aβ1–42 binding to RAGE.
(a) Graph shows binding of Aβ1–42 to RAGE when CAP37 was added simultaneously to wells at increasing concentrations in the presence (inverted triangles) or absence (squares) of protease inhibitors. An IC50 value of 1.28 μM was computed for inhibition of Aβ1–42 binding to RAGE by CAP37. Two separate Kruskal-Wallis tests were performed for Aβ1–42 binding in the presence and absence of protease inhibitors. A Dunn’s multiple comparisons test was performed for each separate Kruskal-Wallis test to compare binding of Aβ1–42 to RAGE in the presence of increasing concentrations of CAP37. Data are mean ± SEM of results. *p<0.05, **p<0.01. (b) Graph shows binding of Aβ1–42 to RAGE when neutrophil elastase was added simultaneously to wells at increasing concentrations in the presence (inverted triangles) or absence (squares) of protease inhibitors. An IC50 value of 4.85 nM was computed for inhibition of Aβ1–42 binding to RAGE by neutrophil elastase in the absence of protease inhibitors. Two separate Kruskal-Wallis tests were performed for Aβ1–42 binding in the presence and absence of protease inhibitors. A Dunn’s multiple comparisons test was performed for each separate Kruskal-Wallis test to compare binding of Aβ1–42 to RAGE in the presence of increasing concentrations of neutrophil elastase. No inhibition occurred in the presence of protease inhibitors. Data are mean ± SEM of results. *p<0.05.