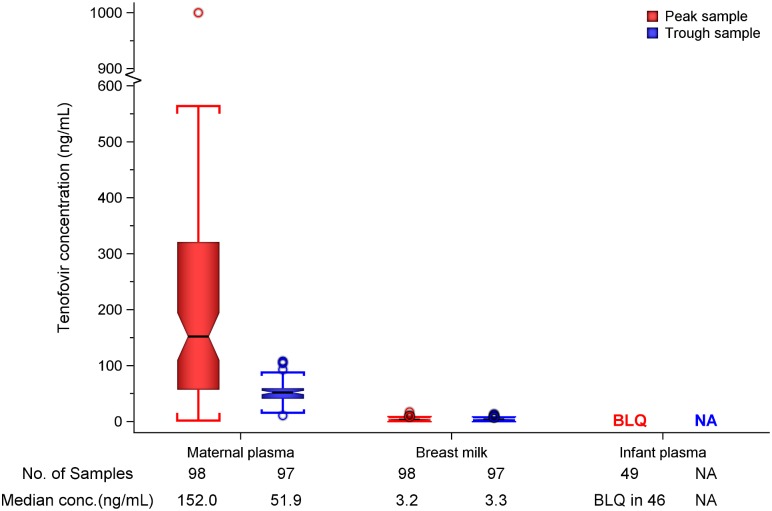
Fig 1. Box plot of maternal and infant tenofovir concentrations.
Non-fasting maternal blood and breast milk samples were obtained concurrently (i.e., within 30 min) at the seventh and tenth visits (corresponding to seventh and tenth maternal DOT PrEP doses). A single infant blood sample was obtained after the seventh maternal DOT PrEP dose. Peak maternal blood, breast milk, and infant blood samples were obtained a median (IQR) of 63 (60 to 68), 70 (65 to 77), and 80 (45 to 90) min after the maternal DOT PrEP dose, respectively. Trough samples were obtained at close of the dosing interval, a median of 23 h (IQR range 23 to 24) after the previous maternal DOT PrEP. One outlier peak maternal plasma tenofovir concentration (1,040 ng/ml) was out of the assay analytic range (0.31–1,000.0 ng/mL). This record was imputed to the upper limit of the assay analytic range and was included in the computation of the displayed summary estimate. Middle box line represents the median. Upper box line represents the 75th percentile and the lower box line represents the 25th percentile. The top whisker denotes the maximum data value or the third quartile plus 1.5 times the interquartile range, whichever is smaller. The lower whisker denotes the minimum data value or the third quartile plus 1.5 times the interquartile range, whichever is larger. The notches display the 95% confidence interval around the median. Small circles represent outlier data points (i.e., observations that are as extreme as ±1.5 of interquartile range). Only 3 of 49 infant plasma samples had quantifiable tenofovir concentration in plasma (infants aged 11 and 13 wk [both 0.9 ng/mL] and 17 wks [17.4 ng/mL]). NA, not applicable; BLQ, below assay limit of quantification for tenofovir: <0.31 ng/mL in plasma and <1 ng/mL in whole milk.