Abstract
Problem
Preterm neonates are highly susceptible to infection. Neonatal host defense against infection seems to be maintained by the temporal presence of immunosuppressive CD71+ erythroid cells. The aim of this study was to investigate whether umbilical cord CD71+ erythroid cells are reduced in neonates born to women who undergo spontaneous preterm labor/birth.
Method of Study
Umbilical cord blood samples (n=155) were collected from neonates born to women who delivered preterm with (n=39) and without (n=12) spontaneous labor, or at term with (n=82) and without (n=22) spontaneous labor. Time-matched maternal peripheral blood samples were also included (n=111). Mononuclear cells were isolated from these samples, and CD71+ erythroid cells were identified and quantified as CD3-CD235a+CD71+ cells by flow cytometry.
Results
1) The proportion of CD71+ erythroid cells was 50-fold higher in cord blood than in maternal blood; 2) a reduced number and frequency of umbilical cord CD71+ erythroid cells were found in neonates born to women who underwent spontaneous preterm labor compared to those born to women who delivered preterm without labor; 3) umbilical cord CD71+ erythroid cells were fewer in neonates born of term pregnancies, regardless of the process of labor, than in those born to women who delivered preterm without labor; and 4) no differences were in umbilical cord CD71+ erythroid cells between neonates born to women who underwent spontaneous preterm labor and those born to women who delivered at term with labor.
Conclusion
Umbilical cord CD71+ erythroid cells are reduced in neonates born to women who had undergone spontaneous preterm labor.
Keywords: Cord blood, immunosuppression, neonates, parturition, placenta, pregnancy
INTRODUCTION
Preterm birth is the leading cause of neonatal morbidity and mortality worldwide; more than 10% of deliveries annually in the U.S. alone are preterm1,2. Two-thirds of preterm births are preceded by spontaneous preterm labor3, a multi-etiological syndrome causally linked to infection and inflammation4. Preterm neonates are at an increased risk for short- and long-term complications5, 6. Indeed, preterm neonates are more susceptible to infection than those delivered at term7, 8. However, the immune mechanisms underlying this susceptibility are poorly understood.
Neonatal susceptibility was originally attributed to the immaturity of the immune system9. However, recent studies are changing this concept. For example, erythroid cells (cells expressing the erythroid-lineage-defining molecule Ter119) are enriched in the neonatal spleen, where they seem to have an immunosuppressive function since they drive Th2 immune responses10. Subsequent in vitro and in vivo studies also demonstrated that neonatal CD71+ erythroid cells have immunosuppressive activity in humans (umbilical cord blood) and mice (the neonatal spleen)11. Such a function is mediated by arginase-2 since supplementation with L-arginine (a substrate for arginase 2) overrides immunosuppression11. Neonatal CD71+ erythroid cells seem to participate in the colonization of commensal microorganisms, which occurs shortly after parturition11. However, this role is still controversial12 since CD71 is highly expressed in the gut epithelium13. Therefore, the enhanced bacterial clearance that occurs upon anti-CD71 treatment may be the result of diminished intestinal barrier function (i.e. immune priming by leaked microbiota) rather than the absence of CD71+ erythroid cells12.
Since CD71+ erythroid cells seem to play a central role in neonatal immunity10, 11 and premature neonates are highly susceptible to infection7, 8, we investigated whether umbilical cord CD71+ erythroid cells were reduced in neonates born to women who underwent spontaneous preterm labor.
MATERIALS AND METHODS
Human subjects, clinical specimens, and definitions
Umbilical cord blood samples were obtained at the Perinatology Research Branch, an intramural program of the Eunice Kennedy Shriver National Institute of Child Health and Human Development, National Institutes of Health, U. S. Department of Health and Human Services (NICHD/NIH/DHHS), Wayne State University (Detroit, MI, USA), and the Detroit Medical Center (Detroit, MI, USA). The collection and utilization of biological materials for research purposes were approved by the Institutional Review Boards of NICHD and Wayne State University. All participating women provided written informed consent. Umbilical cord samples (n=155) were obtained from neonates born to women who delivered at term with (TIL, n= 82) and without (TNL, n=22) spontaneous labor, or preterm with (PTL, n=39) or without (PTNL, n=12) spontaneous labor. The demographic and clinical characteristics of the four groups of women are shown in Table 1. Umbilical cord blood was collected at birth in ethylene-diamine-tetra-acetic acid (EDTA) containing blood collection tubes by venipuncture of the umbilical vein and then transported to the lab for immediate use. Time-matched maternal blood samples were collected when possible (n=111; TNL = 16, TIL = 60, PTNL = 10, and PTL = 25). Labor was defined by the presence of regular uterine contractions at a frequency of at least two contractions every 10 minutes with cervical changes resulting in delivery14. In each case, several tissue sections of the chorioamniotic membranes were evaluated for acute or chronic chorioamnionitis. Also, sections of the umbilical cord were evaluated for phlebitis, arteritis and funisitis. A placental pathology diagnostic was performed according to published criteria15, 16 by pathologists who had been blinded to the clinical outcomes.
Table 1.
Demographics and clinical characteristics of samples used in this study
| TNL (n=22) | TIL (n=82) | PTNL (n=12) | PTL (n=39) | p value | |
|---|---|---|---|---|---|
| Maternal age (y; median [IQR])a | 28 (24-29) | 24 (21-28) | 24 (22-28.3) | 23 (20-27.5) | NS |
| Body mass index (kg/m2; median [IQR])a | 30 (25.5-35.5) | 28 (22-33) | 32.1 (27.6-36.9) | 27.1 (22.9-33) | NS |
| Gestational age at delivery (wk; median [IQR])a | 39 (38.8-39.1) | 39.2 (38.3-40.3) | 33.7 (30.4-35.8) | 35 (33.8-36.3) | p<0.001 |
| Birth weight (g; median [IQR])a | 3447.5 (3090-3801.3) | 3172.5 (2882.5-3650) | 2032.5 (1260-2520) | 2175 (1767.5-2537.5) | p<0.001 |
| Baby Sex (n[%])b | NS | ||||
| Male | 10 (45.5) | 38 (46.3) | 8 (66.7) | 22 (56.4) | |
| Female | 12 (54.5) | 44 (53.7) | 4 (33.3) | 17 (43.6) | |
| Race (n[%])b | NS | ||||
| African-American | 19 (86.4) | 74 (90.2) | 8 (66.7) | 36 (92.3) | |
| Caucasian | 2 (9.1) | 3 (3.7) | 3 (25.0) | 2 (5.1) | |
| Hispanic | 1 (4.5) | 2 (2.4) | 0 (0) | 1 (2.6) | |
| Asian | 0 (0) | 0 (0) | 0 (0) | 0 (0) | |
| Other | 0 (0) | 3 (3.7) | 1 (8.3) | 0 (0) | |
| Primiparity (n[%])b | 2 (9.1) | 15 (18.3) | 1 (8.3) | 9 (23.1) | NS |
| Cesarean section (n[%])b | 22 (100) | 13 (15.9) | 12 (100) | 10 (25.6) | p<0.001 |
| Chronic chorioamnionitis (n[%])b | 10 (45.5) | 26 (31.7) | 5 (41.7) | 12 (30.8) | NS |
| Acute chorioamnionitis (n[%])b | 1 (4.5) | 17 (20.7) | 0 (0) | 8 (20.5) | NS |
| Umbilical Cord Pathology (n[%])b | |||||
| Umbilical Phlebitis | 2 (9.1) | 20 (24.4) | 0 (0) | 5 (12.8) | NS |
| Umbilical Arteritis | 0 (0) | 4 (4.9) | 0 (0) | 5 (12.8) | NS |
| Necrotizing Funisitis | 0 (0) | 0 (0) | 0 (0) | 0 (0) | NS |
Kruskal-Wallis.
X2 test.
IQR, interquartile range.
Cell separation and immunophenotyping
Mononuclear cells were isolated from blood samples (1 ml) by density gradient using Ficoll-Paque Plus (GE Healthcare Biosciences, Uppsala, Sweden) following the manufacturer's instructions. Mononuclear cells were collected from the mononuclear layer of the density gradient, washed with 1ml of 1X phosphate-buffered saline (PBS) (Life Technologies, Grand Island, NY, USA) and immediately used for immunophenotyping. Cells were incubated in 100μl of FACS staining buffer (Cat#554656, BD Biosciences, San Jose, CA, USA) for 30 min at room temperature with the following fluorochrome-conjugated anti-human monoclonal antibodies (BD Biosciences): CD3-BV421(clone UCHT1), CD71-APC (clone M-A712) and CD235a-PE (clone GA-R2). Immediately after staining, cells were incubated with 1X FACS Lysing Solution (BD Biosciences) for 2 minutes at room temperature and washed with FACS staining buffer. Next, cells were resuspended in 0.5 ml of FACS staining buffer and 10μl of CountBright™ absolute counting beads (Life Technologies) were added. Samples were then acquired using a BD FACS LSR II flow cytometer (BD Biosciences). Data were analyzed using the FACSDiva 6.0 software (BD Biosciences). CD71+ erythroid cells were defined as CD3-CD235a+CD71+ cells11. The total number of CD71+ erythroid cells was calculated from the number of acquired CountBright beads. The flow cytometry figures were prepared using FlowJo software version 10 (TreeStar, Ashland, OR, USA).
Statistical analysis
Statistical analyses were performed using SPSS, Version 19.0 (IBM Corporation, Armonk, NY, USA). Normality of the data was tested using the Wilk-Shapiro test. Kruskal-Wallis or Mann-Whitney U- tests were used to compare the medians between groups, and interquartile (IQR) ranges are shown. Comparisons of proportions were made using Χ2 exact tests. A p-value of < 0.05 was used to determine statistical significance. When proportions are displayed, percentages and 95% confidence intervals are shown. Medians are shown with the interquartile range (IQR).
RESULTS
A total of 155 umbilical cord blood samples collected from neonates born to women who delivered preterm or at term gestation were included in this study. Demographic and clinical characteristics of the study population are displayed in Table 1. As expected, the gestational age at delivery was shorter in preterm groups when compared to term groups [PTNL 33.7 GWs (IQR = 30.4-35.8) and PTL 35 GWs (IQR = 33.8-36.3) vs. TNL 39 GWs (IQR = 38.8-39.1) and TIL 39.2 GWs (IQR = 38.3-40.3); p<0.001]. Preterm neonates had a lower birth weight than term neonates [PTNL 2032.5 g (IQR = 1260-2520) and PTL 2175 g (IQR = 1767.5-2537.5) vs. TNL 3447.5 g (IQR = 3090-3801.3) and TIL 3172.5 g (IQR = 2882.5-3650); p < 0.001]. All of the term and preterm deliveries without labor were delivered via C-section.
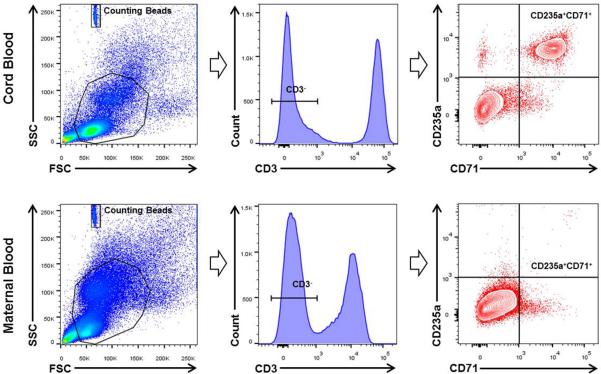
The gating strategy used to determine CD71+ erythroid cells (CD3-CD235a+ CD71+ cells) is shown in Figure 1. Briefly, CD71+ erythroid cells were identified within the CD3- gate since T cells (CD3+ cells) also express the CD71 antigen17. CD71+ erythroid cells also expressed the CD235a antigen, the previously used erythroid marker in umbilical cord blood11. Figure 1 also demonstrates that CD71+ erythroid cells are abundant in umbilical cord blood; yet, are rare in maternal circulation. There were no differences in the frequency and number of maternal blood CD71+ erythroid cells among the four groups (data not shown).
Figure 1.
Gating strategy used to identify CD71+ erythroid cells in umbilical cord blood and maternal peripheral blood. CD71+ erythroid cells (CD235a+CD71+ cells) were identified within the CD3- gate.
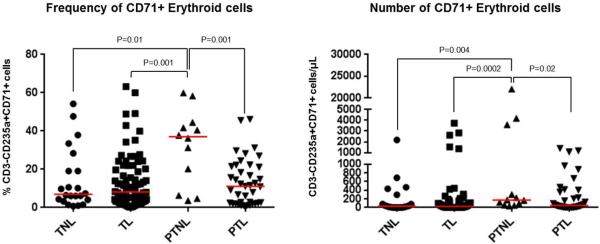
The frequency and number of umbilical cord CD71+ erythroid cells were lower in neonates born to women who underwent spontaneous preterm labor than in those born to women who delivered preterm without labor [PTL 11.10% CD3-CD235a+CD71+ cells (IQR = 2.36-21.5) vs. PTNL 37% CD3-CD235a+CD71+ cells (IQR = 9.76-43.8), p=0.001; and PTL 50.24 cells/μl (IQR = 8.91-356.15) vs. PTNL 170.81 cells/μl (IQR = 68.88-2759.77), p=0.02; Figure 2]. Neither the frequency nor the number of umbilical cord CD71+ erythroid cells was different between the neonates born to women who underwent spontaneous labor at term and those born to women who delivered at term without labor (Figure 2). No differences were observed in the frequency and number of umbilical cord CD71+ erythroid cells in neonates born to women who underwent spontaneous preterm labor compared to those born to women who delivered at term with labor (Figure 2).
Figure 2.
Total frequency and number of CD71+ erythroid cells in umbilical cord blood. Umbilical cord blood were collected from neonates born to women who delivered preterm with (PTL, n=39) and without spontaneous labor (PTNL, n=12), or at term with (TIL, n=82) and without (TNL, n=22) spontaneous labor. Data are shown as scatter plots (median); Mann-Whitney U-tests.
Umbilical cord CD71+ erythroid cells were also more abundant in neonates born to women who delivered preterm without labor than in those born to women who delivered at term with spontaneous labor [PTNL 37% CD3-CD235a+CD71+ cells (IQR = 9.76-43.8) and 170.81 cells/μl (IQR = 68.88-2759.77) vs. TIL 8.16% CD3-CD235a+CD71+ cells (IQR = 3.19-19.82) and 24.32 cells/μl (IQR = 6.43-97.79); p=0.001 and p=0.0002, respectively; Figure 2] or without spontaneous labor [PTNL 37% CD3-CD235a+CD71+ cells (IQR = 9.76-43.8) and 170.81 cells/μl (IQR = 68.88-2759.77) vs. TNL 6.89% CD3-CD235a+CD71+ cells (IQR = 3.88-21.42) and 29.67 cells/μl (IQR = 5.17-134.89); p=0.01 and p=0.004, respectively; Figure 2].
DISCUSSION
Preterm neonates are highly susceptible to infection7, 8. This susceptibility is attributed to the immaturity of multiple immune pathways that are related to arginine depletion18. CD71+ erythroid cells suppress immune responses via arginase-2 activity11. The study herein demonstrates that CD71+ erythroid cells are reduced in the umbilical cord of neonates born to women who underwent spontaneous preterm labor compared to those born to women who delivered preterm in the absence of labor. This data suggests that umbilical cord CD71+ erythroid cells are enriched in preterm gestations, and that the premature process of labor is linked to a reduction in the abundance of these cells. Also, this finding challenges the hypothesis that all preterm neonates have a low frequency of CD71+ erythroid cells, and indicates that the pathological process of labor may reduce immunosuppressive responses in the neonate.
Human CD71+ erythroid cells prevent the activation of other cord blood immune cells in response to heat-killed Listeria monocytogenes11. The depletion of human CD71+ cells in cord blood samples unleashes the release of TNF-α and IL-6, as well as the activation of CD8+ T cells11. Therefore, umbilical cord CD71+ erythroid cells modulate fetal as well as neonatal immune responses11, 19. We found that umbilical cord CD71+ erythroid cells are more abundant in neonates born to women who delivered preterm without labor than in neonates born to women who delivered at term, regardless of the process of labor. These data suggest that umbilical cord CD71+ erythroid cells play a role in both fetal and neonatal immunity. Further research is needed in order to investigate the functional properties of umbilical cord CD71+ erythroid cells in preterm gestations and whether these cells can be detected in earlier stages of pregnancy.
Contrary to the current hypothesis, the study herein demonstrated that umbilical cord CD71+ erythroid cells are not reduced in neonates born to women who underwent spontaneous preterm labor when compared to those born to women who delivered at term gestation with labor. Yet, additional research is needed in order to investigate whether umbilical cord CD71+ erythroid cells display differences in functionality between spontaneous labor at term and preterm labor.
In summary, the study herein demonstrates that umbilical cord CD71+ erythroid cells are reduced in neonates born to women who underwent spontaneous preterm labor when compared to those born to women who delivered preterm without labor. This finding provides insight into the impaired immune mechanisms in premature neonates born to mothers undergoing spontaneous preterm labor. These data also suggest that the premature process of labor alters the abundance of umbilical cord CD71+ erythroid cells, which could be associated with an increased susceptibility to infection.
ACKNOWLEDGEMENTS
This research was supported, in part, by the Perinatology Research Branch, Division of Intramural Research, Eunice Kennedy Shriver National Institute of Child Health and Human Development, National Institutes of Health, U. S. Department of Health and Human Services (NICHD/NIH/DHHS), and, in part, with federal funds from the NICHD/NIH/DHHS under Contract No. HHSN275201300006C. This research was also supported by the Wayne State University Perinatal Initiative in Maternal, Perinatal and Child Health. We thank the physicians and nurses from the Center for Advanced Obstetrical Care and Research, and the research assistants from the PRB Clinical Laboratory, for their help in collecting human samples. We also thank staff members of the PRB Histology and Pathology Units for their examination of the pathological sections, and Tara Mial for her critical readings of the manuscript.
Footnotes
Disclosure/Conflict of Interest
The authors disclose no conflicts of interest.
REFERENCES
- 1.Liu L, Oza S, Hogan D, Perin J, Rudan I, Lawn JE, Cousens S, Mathers C, Black RE. Global, regional, and national causes of child mortality in 2000-13, with projections to inform post-2015 priorities: an updated systematic analysis. Lancet. 2015;385:430–440. doi: 10.1016/S0140-6736(14)61698-6. [DOI] [PubMed] [Google Scholar]
- 2.Martin JA, Hamilton BE, Osterman MJ, Curtin SC, Matthews TJ. Births: final data for 2013. Natl Vital Stat Rep. 2015;64:1–65. [PubMed] [Google Scholar]
- 3.Goldenberg RL, Culhane JF, Iams JD, Romero R. Epidemiology and causes of preterm birth. Lancet. 2008;371:75–84. doi: 10.1016/S0140-6736(08)60074-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Romero R, Dey SK, Fisher SJ. Preterm labor: one syndrome, many causes. Science. 2014;345:760–765. doi: 10.1126/science.1251816. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Mwaniki MK, Atieno M, Lawn JE, Newton CR. Long-term neurodevelopmental outcomes after intrauterine and neonatal insults: a systematic review. Lancet. 2012;379:445–452. doi: 10.1016/S0140-6736(11)61577-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Manuck TA, Sheng X, Yoder BA, Varner MW. Correlation between initial neonatal and early childhood outcomes following preterm birth. Am J Obstet Gynecol. 2014;210:426, e421–429. doi: 10.1016/j.ajog.2014.01.046. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Lawn JE, Cousens S, Zupan J. Lancet Neonatal Survival Steering T: 4 million neonatal deaths: when? Where? Why? Lancet. 2005;365:891–900. doi: 10.1016/S0140-6736(05)71048-5. [DOI] [PubMed] [Google Scholar]
- 8.Goldenberg RL, McClure EM, Saleem S, Reddy UM. Infection-related stillbirths. Lancet. 2010;375:1482–1490. doi: 10.1016/S0140-6736(09)61712-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Kollmann TR, Levy O, Montgomery RR, Goriely S. Innate immune function by Toll-like receptors: distinct responses in newborns and the elderly. Immunity. 2012;37:771–783. doi: 10.1016/j.immuni.2012.10.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Rincon MR, Oppenheimer K, Bonney EA. Selective accumulation of Th2-skewing immature erythroid cells in developing neonatal mouse spleen. Int J Biol Sci. 2012;8:719–730. doi: 10.7150/ijbs.3764. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Elahi S, Ertelt JM, Kinder JM, Jiang TT, Zhang X, Xin L, Chaturvedi V, Strong BS, Qualls JE, Steinbrecher KA, Kalfa TA, Shaaban AF, Way SS. Immunosuppressive CD71+ erythroid cells compromise neonatal host defence against infection. Nature. 2013;504:158–162. doi: 10.1038/nature12675. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Wynn JL, Scumpia PO, Stocks BT, Romano-Keeler J, Alrifai MW, Liu JH, Kim AS, Alford CE, Matta P, Weitkamp JH, Moore DJ. Neonatal CD71+ Erythroid Cells Do Not Modify Murine Sepsis Mortality. J Immunol. 2015;195:1064–1070. doi: 10.4049/jimmunol.1500771. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Matysiak-Budnik T, Moura IC, Arcos-Fajardo M, Lebreton C, Menard S, Candalh C, Ben-Khalifa K, Dugave C, Tamouza H, van Niel G, Bouhnik Y, Lamarque D, Chaussade S, Malamut G, Cellier C, Cerf-Bensussan N, Monteiro RC, Heyman M. Secretory IgA mediates retrotranscytosis of intact gliadin peptides via the transferrin receptor in celiac disease. J Exp Med. 2008;205:143–154. doi: 10.1084/jem.20071204. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.American College of O. Gynecology Committee on Practice B-O: ACOG Practice Bulletin Number 49, December 2003: Dystocia and augmentation of labor. Obstet Gynecol. 2003;102:1445–1454. doi: 10.1016/j.obstetgynecol.2003.10.011. [DOI] [PubMed] [Google Scholar]
- 15.Redline RW. Placental pathology: a systematic approach with clinical correlations. Placenta. 2008;29(Suppl A):S86–91. doi: 10.1016/j.placenta.2007.09.003. [DOI] [PubMed] [Google Scholar]
- 16.Kim CJ, Romero R, Kusanovic JP, Yoo W, Dong Z, Topping V, Gotsch F, Yoon BH, Chi JG, Kim JS. The frequency, clinical significance, and pathological features of chronic chorioamnionitis: a lesion associated with spontaneous preterm birth. Mod Pathol. 2010;23:1000–1011. doi: 10.1038/modpathol.2010.73. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Caruso A, Licenziati S, Corulli M, Canaris AD, De Francesco MA, Fiorentini S, Peroni L, Fallacara F, Dima F, Balsari A, Turano A. Flow cytometric analysis of activation markers on stimulated T cells and their correlation with cell proliferation. Cytometry. 1997;27:71–76. doi: 10.1002/(sici)1097-0320(19970101)27:1<71::aid-cyto9>3.0.co;2-o. [DOI] [PubMed] [Google Scholar]
- 18.Badurdeen S, Mulongo M, Berkley JA. Arginine depletion increases susceptibility to serious infections in preterm newborns. Pediatr Res. 2015;77:290–297. doi: 10.1038/pr.2014.177. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Elahi S. New insight into an old concept: role of immature erythroid cells in immune pathogenesis of neonatal infection. Front Immunol. 2014;5:376. doi: 10.3389/fimmu.2014.00376. [DOI] [PMC free article] [PubMed] [Google Scholar]