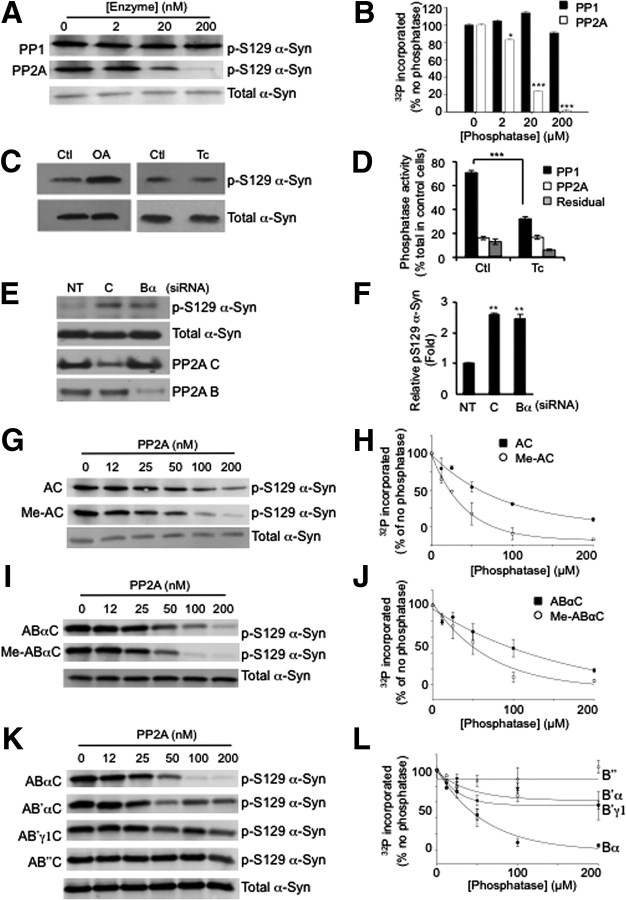
Figure 1.
PP2A catalyzes the dephosphorylation of pS129 α-Syn in a methylation-dependent manner. A, In vitro dephosphorylation reactions performed by incubating phosphorylated α-Syn with PP1 (top) or PP2A (middle) demonstrate that PP2A, but not PP1, dephosphorylates α-Syn in a concentration-dependent manner. B, Quantification of the effects of PP1 and PP2A on α-Syn dephosphorylation. *p < 0.05; ***p < 0.005. C, Western blotting of lysates from SH-SY5Y cells stably expressing α-Syn treated for 1 h with 250 nm okadaic acid (OA) or 1 μm tautomycetin (Tc) with p-S129 α-Syn antibody shows that inhibition of PP2A but not PP1 modulates α-Syn phosphorylation (top). Total levels of α-Syn are unaffected by OA or Tc treatment (bottom). Ctl, Control. D, Tautomycetin is active on PP1 in SH-SY5Y cells. Cells incubated with ethanol (control) or 1 μm Tc for 20 min were lysed and in vitro dephosphorylation reactions were performed using 32P-labeled phosphorylase a. Activities are defined as follows: PP1, the fraction sensitive to 2 μm OA but insensitive to 5 nm OA; PP2A, the fraction sensitive to 5 nm OA; and residual fraction, insensitive to 2 μm OA. ***p < 0.005. E, Knockdown of PP2A subunits results in increased p-S129 α-Syn. SH-SY5Y cells expressing α-Syn were transfected with siRNA of nontargeting (NT), catalytic C subunit, or Bα subunit. Cell lysates were subjected to Western blotting for p-S129 α-Syn, total α-Syn, PP2A C subunit, and PP2A B subunit. Representative data from three separate experiments are shown. F, Quantification of p-S129 α-Syn compared to total α-Syn with knockdown of PP2A subunits. **p < 0.01. G, Western blots with pS129 α-Syn antibody of in vitro dephosphorylation reactions performed by incubating phosphorylated α-Syn with PP2A AC dimer (AC, top) or methylated AC dimer (Me-AC, middle) indicate that methylation increases the ability of PP2A AC to dephosphorylate α-Syn at Ser129. H, Quantification of the effects of methylation of AC dimers on PP2A activity toward phosphorylated α-Syn. I, Heterotrimeric assemblies of PP2A ABαC subunits (ABαC, top) increase activity to dephosphorylate α-Syn subsequent to methylation (Me-ABαC, middle). J, Quantification of the effects of methylation of ABαC heterotrimers on PP2A activity toward phosphorylated α-Syn. K, Different regulatory B subunits strongly influence the ability of PP2A to dephosphorylate α-Syn at Ser129. L, Quantification of the effects of B subunit composition on PP2A activity toward phosphorylated α-Syn.