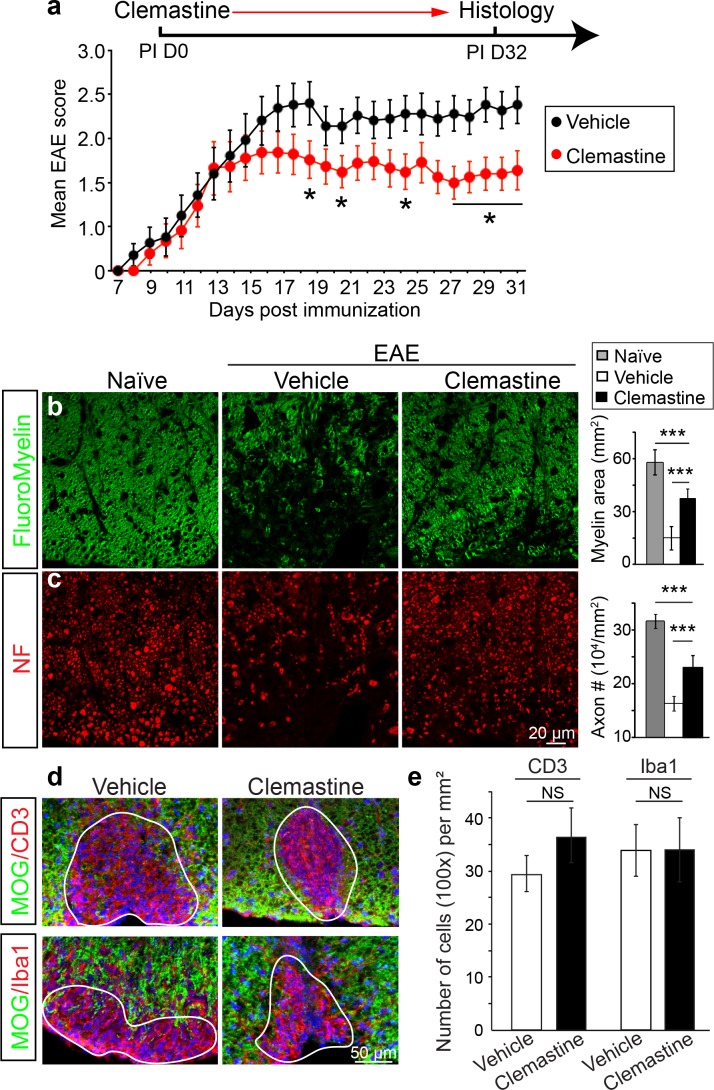
Figure 2. Administration of clemastine attenuates EAE clinical scores and prevents axonal loss.
(a) Daily treatment of clemastine (10 mg/kg, n = 25) from day 0 decreases the clinical severity in the MOG35-55 induced EAE model. Error bars represent mean ± s.e.m. Significance of data is based on the Mann-Whitney test for individual days comparing vehicle (n = 25) to clemastine treated (*p<0.05). (b, c) FluoroMyelin (green, b) and NF immunostaining (red, c) illustrates that clemastine promotes myelin positive staining and prevents axonal loss in the white matter tracts of the spinal cord cross sections compared to the vehicle control. FluoroMyelin positive areas and NF positive axons were quantified. Error bars represent mean ± s.e.m. and all experiments were performed in triplicate. ***p<0.001, significance based on Student’s t-test with the respective controls. n = 3 for all experiments. (d) Demyelinated lesions from spinal cord sections from clemastine treated and vehicle control mice were analyzed for T-cells and macrophages/microglia. Sections were immunostained for MOG (green), CD3 (T-cells; red), and Iba1 (macrophages and microglia; red). (e) Density of CD3 and Iba1 positive cells were quantified in demyelinated lesions from vehicle control and clemastine treated mice at the late stage of EAE. Error bars represent mean ± s.e.m. (experiments were performed in triplicate).