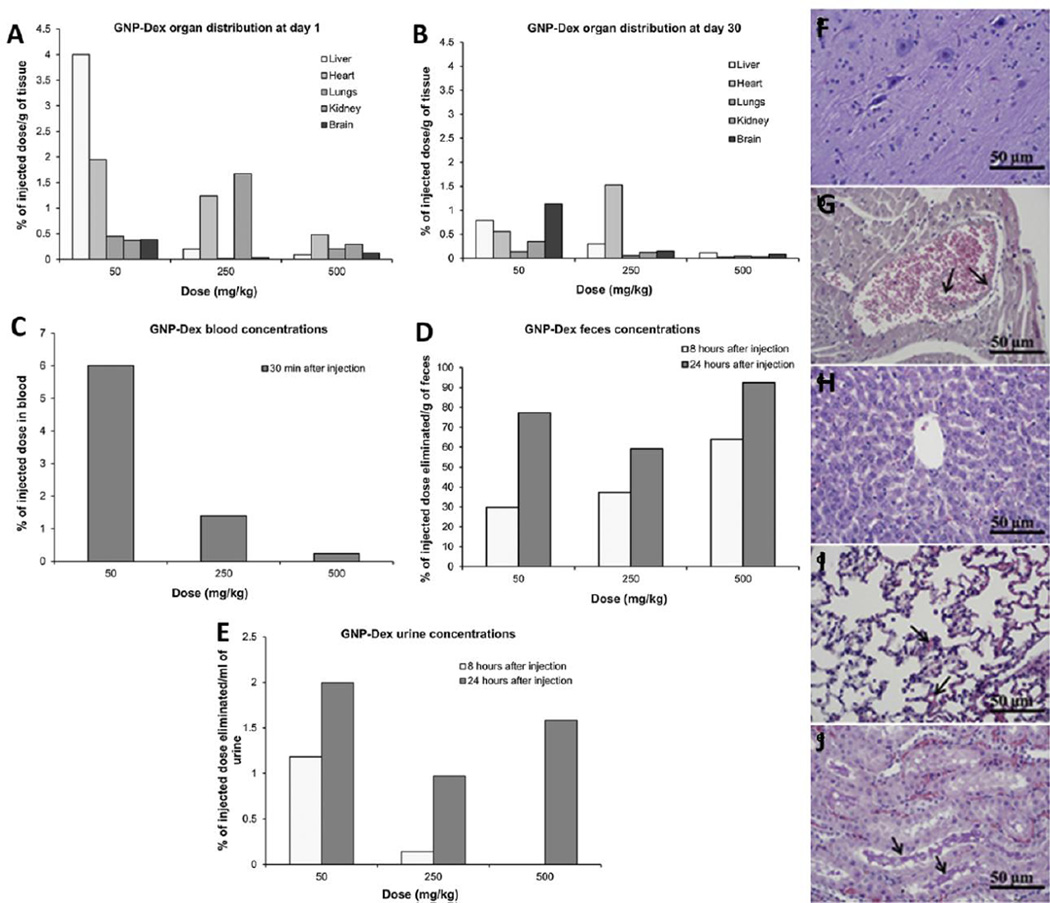
Figure 12.
(A&B) Tissue biodistribution, (C) blood half life, (D) elimination via feces and (E) urine after GNP-Dex administration at doses 50–500 mg/kg to Wistar rats analyzed via ICP-MS. Liver and kidney showed maximum uptake after 24 hours of administration. Majority of GNP-Dex was excreted via feces; small amounts were cleared via urine. Histological sections of (F) cerebral cortex, (G) myocardium, (H) liver, (I) pulmonary parenchyma and (J) renal cortex after 24 hours of GNP-Dex administration at 250 mg/kg dose. No diagnostic abnormalities were observed in cerebral cortex and liver. Vascular congestion of myocardium was observed. Arrows in (G) show dilated vein containing debris of GNP-Dex. Mild focal congestion was observed in the alveolar capillaries of pulmonary parenchyma. Vascular congestion and proteinaceous casts were observed in renal tubules of renal cortex. Adapted from Reference [104] with permission, copyright © Elsevier, 2014.