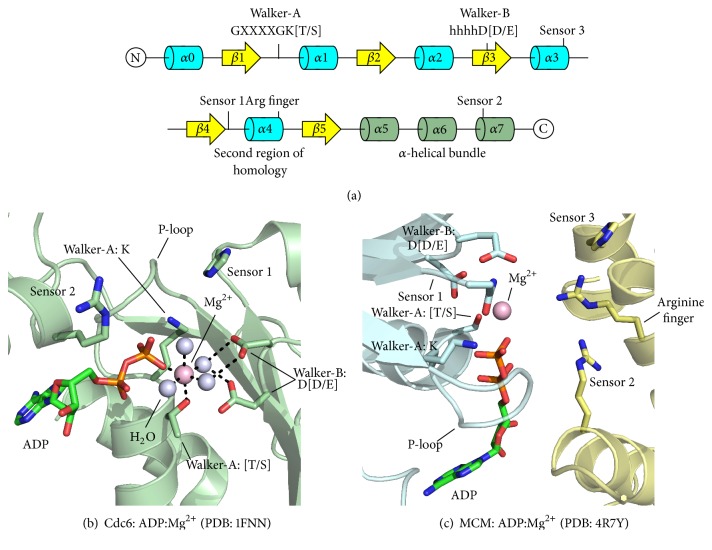
Figure 1.
Features of the AAA+ ATPase domain. (a) The AAA+ α-β-α fold topology and active site feature locations are shown in primary sequence and secondary structure. Helices and strands within the core α/β fold are colored in blue and yellow, respectively. C-terminal lid domain helices are colored in light green. (b) Active site residues are precisely positioned to bind nucleotide and Mg2+. The Mg2+ cation is directly coordinated by the Walker-A threonine, the β-phosphate of the bound nucleotide, and four water molecules. Dashed lines indicate discussed molecular interactions (see text). The bound ADP molecule and critical active site features are shown in stick, water molecules as light blue spheres, and the magnesium ion as a magenta sphere. (c) The ATPase site forms at subunit interfaces by residues of adjacent subunits. The Walker-A, Walker-B, and Sensor 1 residues are positioned on the left side of the site and all reside on the same subunit (blue, “cis-acting”), while three basic residues are located on the right side of the site from the neighboring subunit (yellow, “trans-acting”). Bound ADP and Mg2+ are represented identical to (b). The protein topology cartoon in (a) was prepared using the TopDraw software package [42]. All structure representations in the figure were prepared with the Pymol software package [43] and PDB accession codes 1FNN [15] (b) and 4R7Y [41] (c).