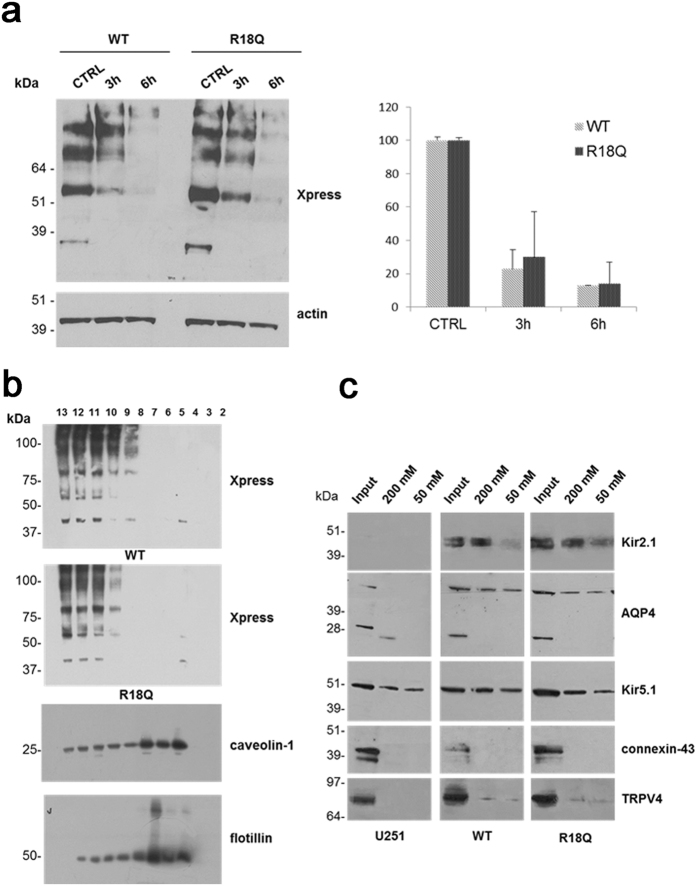
Figure 3. Degradation kinetics, membrane compartmentalization and molecular interactions of WT and R18Q Kir4.1.
(a) Left panel: WB analysis of protein extracts obtained from cells expressing WT and R18Q Kir4.1 channels treated with the protein synthesis inhibitor cycloheximide (CHX) for 3 and 6 h to inhibit protein synthesis and evaluate protein degradation kinetic. Anti-Xpress epitope Ab was used to detect WT and mutant Kir4.1. Actin is used as loading control. Molecular weight markers are on the left (kDa). Right panel: densitometric analysis of WT or R18Q Kir4.1 protein bands after CHX treatment, normalized to the corresponding untreated controls, indicates no significant differences in the degradation kinetics between WT and R18Q Kir4.1 proteins. Data are expressed as mean ± SEM from three independent experiments. (b) WB analysis of cholesterol-rich (Triton insoluble fractions: 4–7) and cholesterol-poor membrane fractions (Triton soluble fractions: 10–13) obtained from WT or R18Q Kir4.1 expressing cells shows that WT and mutant Kir4.1 channels are both distributed in cholesterol-poor membrane fractions. Caveolin-1 and flotillin identify the caveolar raft fractions in cells expressing Kir4.1 WT. Molecular weight markers are on the left (kDa). (c) WB analysis of Kir4.1 channel interactors selected by Histidine (His) affinity chromatography. Eluates derived from astrocytoma cells infected with the empty vector (U251) were used as controls for unspecific binding to NiNTA-resin. The Input lane indicates the total protein extracts before His pull-down assay and 200 mM and 50 mM the proteins eluted from NiNTA-resin using imidazole (50, 200 mM, respectively). Kir2.1, aquaporin-4 (AQP4), Kir5.1, TRPV4, but not connexin-43 were found among the proteins co-eluted with Kir4.1. No difference was observed between WT and R18Q Kir4.1 interactors. One representative experiment out of four is shown. Molecular weight markers are indicated on the left (kDa).