Abstract
The primary cilium, a sensory organelle, regulates cell proliferation and neuronal development of dentate granule cells in the hippocampus. However, its role in the function of mature dentate granule cells remains unknown. Here we specifically depleted and disrupted ciliary proteins IFT20 and Kif3A (respectively) in mature dentate granule cells and investigated hippocampus-dependent contextual memory and long-term plasticity at mossy fiber synapses. We found that depletion of IFT20 in these cells significantly impaired context-dependent fear-related memory. Furthermore, we tested synaptic plasticity of mossy fiber synapses in area CA3 and found increased long-term potentiation upon depletion of IFT20 or disruption of Kif3A. Our findings suggest a role of primary cilia in the memory function of mature dentate granule cells, which may result from abnormal mossy fiber synaptic plasticity. A direct link between the primary cilia of mature dentate granule cells and behavior will require further investigation using independent approaches to manipulate primary cilia.
Cilia are hair-like structures composed of microtubules, which can be classified as either motile or primary based on their movement and microtubule structure1. In the mammalian brain, each of various cell types, such as stem/progenitor cells, neurons and glial cells, expresses a single primary cilium, which is generally involved in cell signaling2,3. Several studies of neuronal primary cilia have focused on cell proliferation, and a few recent ones on neuronal migration during embryonic brain development4,5,6. We recently found that almost all developing adult-born dentate granule cells (DGCs) form primary cilia immediately preceding the period of glutamatergic synapse formation7, corresponding to their electrophysiological and functional maturation8. Moreover, these primary cilia are essential for proper integration of DGCs into the existing circuit9. That most adult and embryonically born DGCs retain primary cilia led us to speculate that primary cilia may be important in hippocampus-dependent behavior.
Moreover, mutations in ciliary proteins that cause structural or functional abnormalities are implicated in a wide variety of diseases known as ciliopathies, and defects in primary cilia can lead to neurological impairments such as intellectual developmental disorders and cognitive deficits10,11,12. Previous research has suggested that the pathological phenotypes of ciliopathies might be due to dysfunction in primary cilia-associated signaling pathways during development5,13,14. However, whether neuronal primary cilia have any direct physiological role in regulating neuronal activity or behavior is largely unknown.
Here, we examined the physiological and behavioral function of primary cilia in mature DGCs. We conditionally ablated intraflagellar transport (IFT) genes, IFT20 or expressed a dominant negative form of Kinesin family member 3A (Kif3A) under the control of the CaMKII promoter using adeno-associated viruses (AAV). We found that ablation of IFT20 in mature DGCs led to impaired hippocampus-dependent contextual memory. We further found that IFT20 ablation or Kif3A disruption in mature DGCs enhanced long-term potentiation (LTP) induced at mossy fiber (MF) synapses of these cells.
Materials and Methods
Mice
Conditional IFT20 mutant mice were kindly provided by Dr. Gregory J Pazour at the University of Massachusetts Medical School15. All mice used in experiments were housed under a 12-hour light/dark cycle. All animal procedures were conducted in accordance with the institutional animal guidelines and were approved by the ethical committee of the State University of New York at Stony Brook.
Manipulation of mature dentate granule cells
High titer engineered AAV9 under the CaMKII promoter was purchased from the inventory of vector core facility from University of Pennsylvania. AAV9-DIO-dnKif3A-dTomato was produced as described previously9. High titer AAVs were stereotaxically injected into the dentate gyrus of ~5–6 week-old adult mice. To allow for efficient viral transduction and maturation of potentially labeled newborn DGCs16, mice were used for experiments 14 days after AAV injection.
Immunohistochemistry and quantification
Mice were anesthetized and transcardially perfused with PBS and 4% PFA. Fixed brains were sliced by a sliding microtome, creating 40 μm thick coronal brain sections.
Prior to primary antibody incubation, brain sections were blocked with 10% donkey serum in 0.25% Triton-PBS for 1 hour. Next, sections were incubated overnight at 4 °C with diluted primary antibodies (rabbit anti-adenylyl cyclase III 1:300, Santa Cruz Biotechnology, goat anti-GFP 1:500, Rockland, goat anti-DCX 1:500, Santa Cruz Biotechnology, mouse anti-parvalbumin, 1:1000, Sigma). The next day, brain sections were incubated in diluted secondary antibodies solution (mouse anti-goat AlexaFluoro 488 1:1000, Jackson Labs, Donkey anti-rabbit CY-3 1:1000, Jackson Labs, donkey anti-rabbit AlexaFluoro 647 1:1000, Jackson Labs, donkey anti-mouse Cy-3, 1:1000, Jackson Labs) for 2 hours at room temperature. Sections were mounted on slides using DAPI-containing mounting media to stain nuclei. All images were acquired on an Olympus FV1000 confocal system. For DCX + cell quantification, randomly selected, 40 μm-thick, z-stacked images were collected across the entire anterior/posterior axis of the dentate gyrus (field side 320 μm × 320 μm; 60 fields from 3 control mice, 72 fields from 4 IFT20 (−/−) mice), and 2 μm guard zones were used to avoid edge artifacts. Dendrite morphology was analyzed using the Sholl analysis function of the NeuronJ plug-in for ImageJ.
Cilia were identified and related to their cell of origin for quantification using an anatomical- and landmark-based approach4,9. Briefly, each mature DGC lines up perpendicular to the GCL and extends a single primary cilium radially in the same direction and in the immediate vicinity of its primary apical dendrite. Moreover, the primary cilium, which is thickest at its base, arises from the mother centriole, lying immediately superior to the nucleus in the DGC. Based on these features, 3-dimensionally reconstructed z-stacked images were used to identify all cells expressing primary cilia (DAPI + /ACIII+), as well as virus-labeled cells expressing primary cilia (by overlaying GFP).
Analysis of parvalbumin and GFP co-localization was conducted by taking 40 μm-thick, z-stacked images across the entire anterior/posterior axis of the dentate gyrus (field side 320 μm × 320 μm). Co-localization was confirmed by overlaying GFP in 3-D reconstructed images of parvalbumin + cells in subgranular zone and hilus of the dentate gyrus.
Contextual fear conditioning (CFC)
We conducted the procedure for CFC as we have previously described16. Mice were placed in a fear-conditioning chamber consisting of transparent front and back walls, stainless steel sidewalls and a stainless steel shock grid floor (18 × 18 × 30 cm, Coulbourn). In fear context A, 70% ethanol was used to remove any odor for the context before each experiment. During training in context A, mice were allowed to explore the context for 2 minutes before a 30 second tone (2800 Hz, 85 dB) followed by a 2 second foot shock (0.5 mA). Mice were removed from the conditioning chamber 30 seconds after the foot shock and transferred back to their home cages. A probe test for contextual fear memory was conducted 24 hours after training. Mice were re-exposed to context A without tone or foot shock, and freezing levels during a 5-minute period were measured. A probe test for toned fear memory was conducted 4 hours after the previous probe test. Mice were placed into a novel context B, which consisted of white plastic surrounding walls and a covered floor without an odor cue. A tone was presented for the last 3 minutes of the 5-minute probe test to evaluate tone-conditioned fear memory, and freezing level during this probe test was measured. Mice movements were recorded using Freeze Frame software, and freezing levels were analyzed by Freeze View software with 1-second minimum bout duration.
Pre-exposure contextual fear conditioning (PECFC)
PECFC was conducted to probe pattern memory encoding on the basis of partial cue information from a fear context to which an animal was pre-exposed. We used an experimental paradigm based on previous work17. Briefly, on day 1, mice were allowed to freely explore in a conditioning chamber, same as described in CFC test, for 10 minutes. Twenty-four hours after the pre-exposure, mice were re-exposed to the context and received a foot shock 10 seconds after entering the context. A probe test was conducted on day 3 by analyzing the freezing level of mice during a 5-minute exposure period to examine the expression of a previously formed fear memory based on partial cues from the pre-exposed context.
Contextual Fear Discrimination Test
We used a contextual fear discrimination test adapted from previous work18. On day 0, mice received a foot shock at context A to generate a contextual fear association. This context contains a stainless steel grid floor, transparent front and back walls with a mild alcohol scent for the olfactory cue. This context was cleaned with 70% ethanol before each mouse was placed in the context. For fear memory learning, mice received a 2 second single foot shock (0.5 mA) at 185 seconds after placement into the chamber. Mice were removed from the chamber and returned to home cages 15 seconds after the foot shock termination.
For the next 8 consecutive days, mice were exposed to context A (fear context) and a similar context, context B (safe context in which no shock was delivered). Context B consisted of a stainless steel grid floor as in context A, but contained black and white striped front and back walls and lacked an olfactory cue. This context was cleaned with odorless non-alcoholic antiseptic solution before each mouse was placed in the context. Mice were allowed to explore freely for 180 seconds without any shock. The order of exposure on each day was AB/AB/BA/BA/AB/BA/AB and there was a 4-hour gap between exposures to each context.
All mice movements were recorded through FreezeFrame software and percentage of freezing was analyzed by FreezeView software with 1 second of minimum bout duration. Freezing levels during the first 180 seconds of each session were averaged for each group and used to calculate discrimination ratios;
Discrimination Ratio = (Freezing Context A – Freezing Context B)/(Freezing Context A + Freezing Context B).
Spatial novelty recognition test
We performed the spatial novelty recognition test similar to what has been previously described19. On days 1–3, mice were habituated to an open arena (50 cm3 opaque box) lacking objects. On day 4, mice were exposed to two identical objects arranged in one positioning pattern for 10 minutes. On day 5, one of the two objects was moved to a novel location. Object location zones were defined and animal paths were recorded using EthoVision XT video tracking software.
Elevated Plus Maze (EPM)
The EPM is a paradigm used for testing anxiety-like behavior in rodents20. The apparatus used for the EPM has two open arms and two closed arms surrounded by transparent plastic walls, and stands 50 cm above the floor. To test anxiety-like behavior, mice were placed at the center of the apparatus and allowed to explore freely for 5 minutes. The time spent in each arm and the center was measured and analyzed, with longer time spent in the open arms interpreted as corresponding to lower anxiety-like states.
Light/Dark transition test
The light/dark transition test measures anxiety-like behavior associated with rodents’ innate light avoidance. The apparatus has two connecting compartments- a light compartment that has transparent plastic walls and was illuminated during experiment, and a dark compartment that is covered with black and opaque plastic walls. Mice were placed in the dark compartment and allowed to freely explore both compartments for 10 minutes. Latency to enter the light compartment and time spent in each compartment were measured.
Electrophysiology
Preparation of brain slices and electrophysiological recordings was performed as previously described16,21. To examine synaptic plasticity of the MF tract, we placed a stimulating electrode at the MF tract near the dentate gyrus and a recording electrode in area CA3. Field excitatory postsynaptic potentials (fEPSPs) were recorded with an intensity that produced a half maximal response. High frequency stimulation (HFS) (100 stimuli at 100 Hz) was delivered to induce synaptic plasticity. The average of fEPSPs slope in every 4 sweeps was analyzed. LTP was determined by comparing the averaged fEPSPs slope of the baseline (-10-0 minutes) and the last 10-minute (50–60 minutes).
Statistical analysis
Data were analyzed using two-tailed unpaired Student t-tests, repeated measures ANOVAs, and two-way ANOVAs. The cut-off for statistical significance was set at a p value of less than 0.05. All data are presented as mean ± SEM.
Results
Depletion of primary cilia from mature dentate granule cells
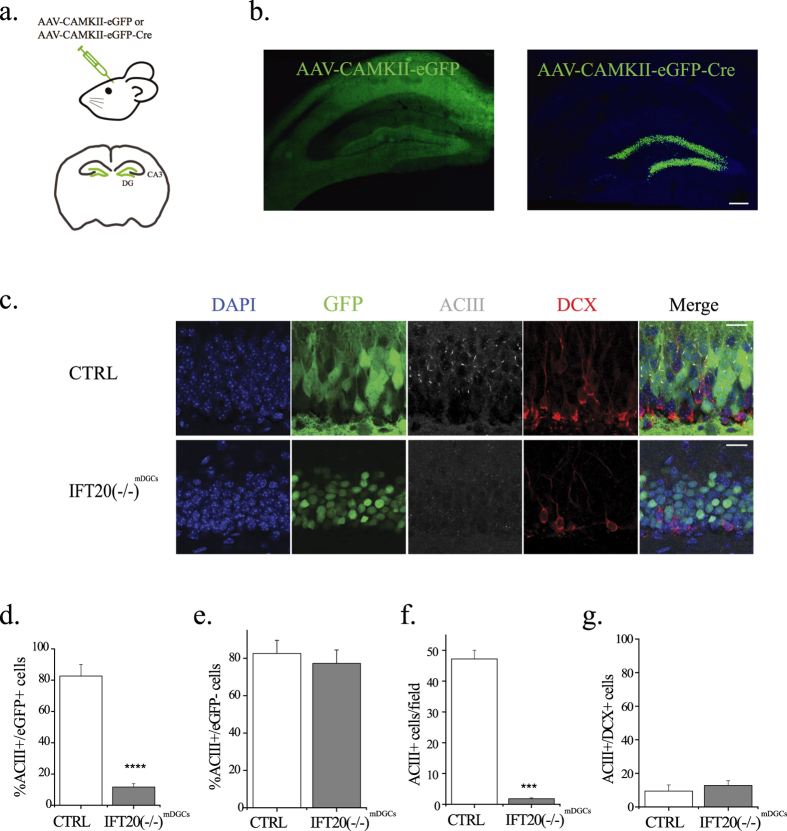
We set out to examine the physiological and behavioral role of primary cilia in mature DGCs by specifically depleting IFT20 in these cells. AAV9 expressing Cre recombinase under the control of the CaMKII promoter (or eGFP under the CaMKII promoter as control) was stereotaxically injected into the dentate gyrus of adult floxed IFT20 mice22 (Fig. 1a). Two weeks after the injection, we observed extensive transduction of AAV along the entire anterior/posterior axis of the dentate gyrus with the expression of the CaMKII promoter driven Cre recombinase, with 86.3 ± 3.2% of cells in the granule cell layer expressing Cre (as seen by eGFP positivity), or eGFP alone, with 89.4 ± 7.0% of cells in the granule cell layer labeled with eGFP in the control condition (Fig. 1b,c). We noted that in the control group there was wide spread of the GFP signal into other hippocampal regions. This may result from better diffusion of the AAV-eGFP, which remains to be determined. However, labeling in the Cre group was much more specific to the DG because Cre-eGFP was constrained to the nucleus of infected cells in the experimental condition (Fig. 1b). To confirm the specificity of viral transduction to the granule cell population, we co-stained transduced cells for parvalbumin (PV), a marker of the major population of interneurons in the DG, in mice injected with AAV-CaMKII-eGFP and AAV-CAG-eGFP. We observed no overlap between PV+ cells and GFP+ cells in the CaMKII group (0/100 cells). As expected, as a comparison, the AAV-CAG (a generic promoter)-eGFP injected group exhibited ~27% of PV+ cells that co-localized with GFP (14/51 cells) (Supplementary Fig. 1).
Figure 1. Depletion of primary cilia from mature dentate granule cells.
(a) Schematic diagram of the AAV injection in the dentate gyrus. (b) Representative confocal images of the hippocampus of IFT20 fl/fl mice transduced with AAV-CaMKII-eGFP or AAV-CaMKII-eGFP-Cre virus at 28 days post AAV labeling. Scale bar: 200 μm. (c) Representative confocal images of the granule cell layer of AAV-CaMKII-eGFP or AAV-CaMKII-eGFP-Cre 28 days post injection. Immunostaining with anti DCX (red) and ACIII (white) reveals that primary cilia were selectively ablated in mature DGCs by AAV-CaMKII-eGFP-Cre transduction, whereas no ciliary disruption was found by AAV-CaMKII-eGFP transduction. Scale bars: 30 μm (d) AAV-CaMKII-eGFP-Cre transduced cells exhibited a significantly depressed rate of ACIII expression versus AAV-CaMKII-eGFP controls. (Cre: 11.7 ± 2.1% ACIII+, eGFP: 82.6 ± 7.4% ACIII+; two-tailed un-paired t-test p = 8.5 × 10−7). (e) Cells in the granule cell layer that did not take up virus expressed primary cilia at comparable rates between eGFP control and Cre groups (CTRL: 82.6 ± 7.0%; Cre: 77.3 ± 7.2%; two-tailed unpaired t-test p = 0.634; n = 3,3). (f) Cre transduction resulted in significant gross depletion of primary cilia. (Averaged number of ACIII positive cells per field in CTRL and IFT20(−/−)mDGCs: 47.23 ± 2.78 for CTRL and 1.8 ± 0.28 for IFT20(−/−)mDGCs; p = 5.985 × 10−15; n = 4,4). (g) Expression of ACIII in DCX+ cells did not differ significantly between eGFP-Cre and eGFP controls (CTRL: 9.5 ± 3.6% co-localization; IFT20(−/−)mDGCs : 12.8 ± 2.9% co-localization; two-tailed unpaired t-test p = 0.50). Fields size: 320 μm × 320 μm × 36 μm. ***p < 0.001; ****p < 0.0001; n is the number of animals.
To confirm sufficient ablation of primary cilia in AAV-CaMKII-eGFP-Cre-infected cells, we quantified the number of GFP+ cells harboring the ciliary protein ACIII. As expected, the vast majority of Cre+ cells lacked expression of ACIII, compared to the proportion of eGFP+ cells expressing ACIII in control mice (Fig. 1d). By contrast, the small minority of cells in the granule cell layer that were Cre negative (13.7 ± 3.2%) expressed ACIII at levels comparable to those observed in the small minority (10.6 ± 7.0%) of eGFP negative control cells (Fig. 1e). Likewise, the total number of ACIII+ cells was dramatically reduced in Cre-expressing animals (IFT20(−/−)mDGCs) versus eGFP-controls (Fig. 1f). These results demonstrate the efficacy of our cilia ablation approach.
Given that the adult hippocampus continuously generates new dentate granule cells23,24,25 and that the CaMKII promoter becomes active in adult-born, mature neurons26, to confirm primary cilium depletion was restricted to the population of mature DGCs in designed experiments, we immunostained hippocampal sections with doublecortin (DCX), a marker of immature neurons27. Two weeks post injection, we observed no overlap between DCX+ and Cre-GFP+ cells in the dentate granule cell layer (Fig. 1c). To confirm that primary cilia ablation was restricted to mature but not young DGCs, we analyzed co-expression of DCX and ACIII and found no significant difference in ACIII expression between IFT20(−/−)mDGCs and controls (Fig. 1g). To determine whether there was any effect of cilia depletion in mature DGCs on hippocampal neurogenesis, we counted DCX+ cells in the granule cell layer and analyzed their dendritic morphology. We found that the number of DCX+ cells was similar between the Cre and GFP only expressing groups, and that there were no significant differences in dendrite morphology as measured by Sholl analysis, suggesting that cilia depletion in mature DGCs did not grossly disrupt adult neurogenesis (Supplementary Fig. 2). Together, our results indicate that AAV-mediated Cre expression in IFT20 floxed animals is an efficient method for ciliary deletion from a large portion of mature DGCs, which is likely sufficient for testing behavioral and physiological functions of primary cilia.
Depletion of primary cilia from mature DGCs impaired hippocampus-associated contextual fear memory and spatial memory but not pattern discrimination or anxiety-like behaviors
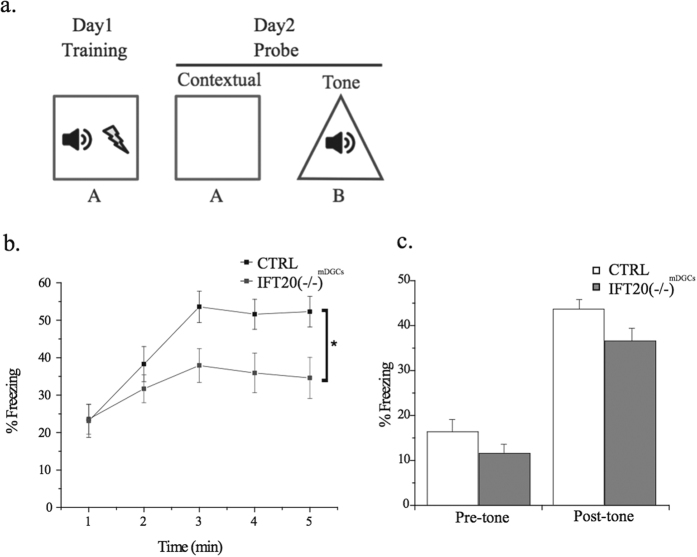
To examine whether depletion of primary cilia from mature DGCs affects hippocampal behaviors, we focused on testing memory by performing a contextual fear memory test. We arranged two groups of animals as described in Fig. 1 and performed the CFC test as illustrated in Fig. 2a. We found that the freezing level in context A was significantly lower in IFT20(−/−)mDGCs as compared to CTRL mice (Fig. 2b). However, IFT20(−/−)mDGCs mice did not differ significantly from CTRL mice on tone-cued freezing in context B (Fig. 2c), suggesting a specific defect in contextual fear memory. This set of results indicates that primary cilia in mature DGCs are involved in normal contextual fear but not in tone-cued fear memory.
Figure 2. Primary cilia ablation from mature dentate granule cells impairs contextual fear memory.
(a) Experimental design for CFC test. (b) IFT20(−/−)mDGCs mice showed a decreased freezing response on the CFC test (repeated measures ANOVA main effect of condition: F(1,16) = 6.979, p = 0.018; n = 17,19). (c) IFT20(−/−)mDGCs and CTRL mice did not differ significantly on the tone-cued fear memory test (two-way ANOVA main effect of condition: F(2,69) = 2.444; p = 0.094; n = 17,19). *p < 0.05; n is the number of animals.
The impairments in CFC observed after the depletion of cilia from mature DGCs, together with a prior study demonstrating that these neurons mediate hippocampus-dependent contextual pattern memory28, led us to speculate that depletion of primary cilia from mature DGCs may affect pattern memory encoding. We therefore performed the PECFC test as previously described17. The experimental procedure for these tests is shown in Fig. 3a. Interestingly, we found that IFT20(−/−)mDGCs mice exhibited significantly less freezing behavior as compared to CTRL mice (Fig. 3b). This finding suggests a role of primary cilia in mature DGCs in pre-exposed contextual memory.
Figure 3. Ablation of primary cilia from mature dentate granule cells impairs pre-exposure contextual fear memory.
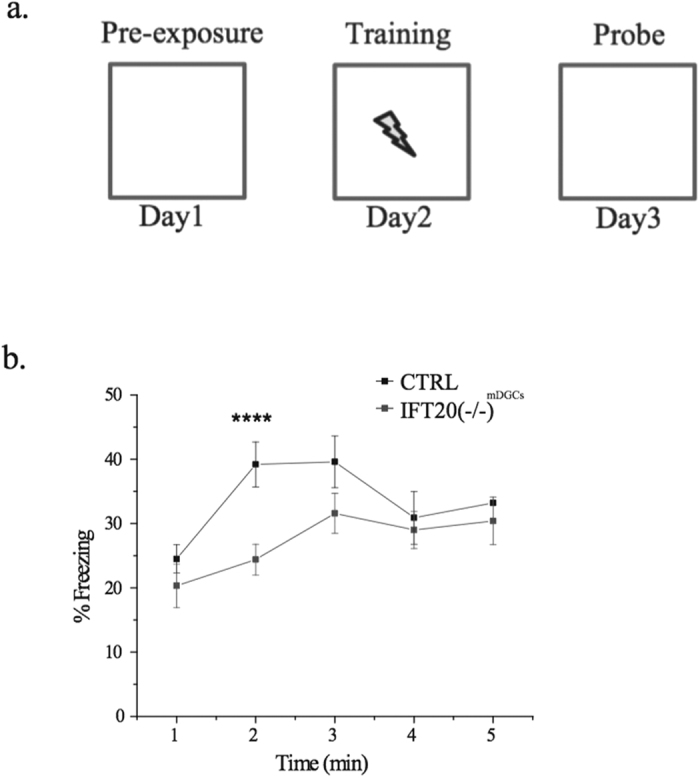
(a) Experimental design for PECFC test. (b) IFT20(−/−)mDGCs showed a reduced freezing response as compare to CTRL when re-exposed to a fear context (repeated measures ANOVA interaction between condition and time F(1, 25) = 4.747, p < 0.0001, followed by LSD comparison at 60–120 sec: p = 4.41 × 10−5; n = 22,23). ****p < 0.0001; n is the number of animals.
We next explored the role of primary cilia in another DG-dependent contextual memory task, pattern encoding, the ability to discriminate distinct representations between similar contexts/episodes18,29,30,31. To examine whether primary cilia in mature DGCs are necessary for pattern encoding, we performed a contextual discrimination task. We found that IFT20(−/−)mDGCs mice did not differ significantly from controls in terms of their ability to discriminate between two similar environments, indicating that primary cilia are not involved, at least directly, in pattern encoding (Supplementary Fig. 3).
In addition to its role in contextual memory, the hippocampus is highly involved in spatial information processing and memory32. To examine whether primary cilia in mature DGCs are necessary for spatial memory expression, we performed the spatial novelty recognition test. We found that unlike CTRL mice, IFT20(−/−)mDGCs mice did not show a significant preference for an object at a novel location, as measured by exploration time at either a novel or familiar location (Supplementary Fig. 4). This suggests that primary cilia in mature DGCs are important for spatial memory expression.
The hippocampus is also known to be involved in anxiety- and depression-associated behaviors33,34,35. To investigate a possible ciliary function in these behaviors, we performed the elevated plus maze (Supplementary Fig. 5) and light/dark transition tests (Supplementary Fig. 6) after depleting primary cilia from mature DGCs. We found that IFT20(−/−)mDGCs mice did not exhibit significant behavioral differences on either test as compared to CTRL mice, suggesting that primary cilia are not directly involved in emotional behaviors, at least as measured by these specific tests.
Ablation of primary cilia from mature DGCs augmented long-term potentiation induced at mossy fiber synapses
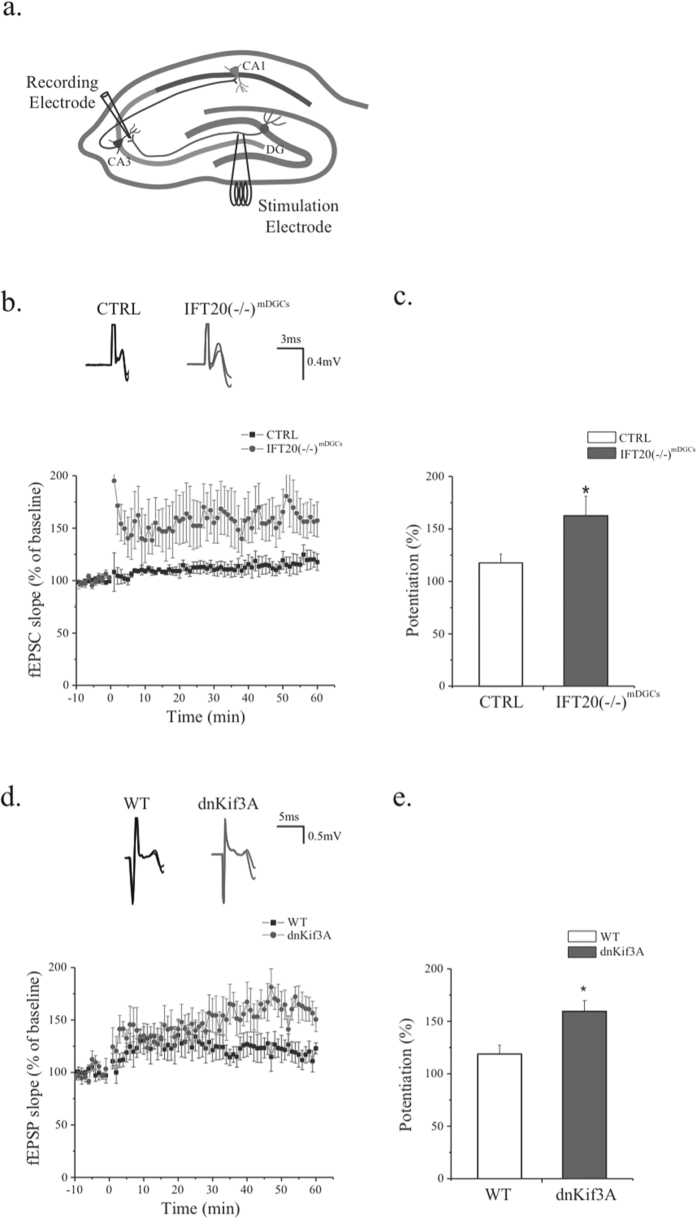
The defect in contextual memory observed after depletion of cilia in mature DGCs suggests a potential impairment in the function of MF synapses of these neurons, as mature, adult-born DGCs contribute importantly to LTP at these synapses36,37. We therefore recorded fEPSPs and studied plasticity by inducing LTP in area CA3 of acutely prepared hippocampal slices while stimulating the MFs (Fig. 4a). To determine whether baseline synaptic transmission was affected we performed an input-output (I-O) analysis of fEPSPs in CTRL and IFT20(−/−)mDGCs mice, showing similar I-O curves as stimulation intensity was increased from 10 to 35 mA (Supplementary Fig. 7). We next induced LTP by high frequency stimulation as we previously described16. Interestingly, MF synapses in the CA3 of IFT20(−/−)mDGCs mice exhibited increased LTP as compared to those of CTRL mice (Fig. 4b). The amplitude of potentiation measured from 50 to 60 minutes after the stimulation was 117.75 ± 8.25% for the CTRL group and 163.69 ± 18.59% for the IFT20(−/−)mDGCs group (p = 0.048; n = 7,8; two-tailed unpaired t-test; Fig. 4c).
Figure 4. Removal of primary cilia from mature dentate granule cells increases synaptic plasticity in the mossy fiber pathway.
(a) A schematic representation of the tri-synaptic circuit and the position of stimulating and recording electrodes. (b) Top, representative traces of both CTRL and IFT20(−/−)mDGCs recorded before and after HFS. Bottom, IFT20(−/−)mDGCs show increased LTP compare to CTRL. (c) Average potentiation of fEPSPs slope during 50–60 minute showing enhanced LTP in IFT20(−/−)mDGCs (two-tailed unpaired t-test p = 0.048; n = 7,8). (d) Top, representative traces of both WT and dnKif3A recorded before and after HFS. Bottom, dnKif3A show increased LTP compare to WT. (e) Average potentiation of fEPSPs slope during 50–60 minute showing LTP enhancement in dnKif3A (two-tailed unpaired t-test p = 0.048; n = 5, 4). *p < 0.05; n is the number of animals.
Several recent studies have shown that IFT20 potentially has non-ciliary functions in certain cell types38,39,40. To confirm that the enhanced LTP after cilia depletion was indeed due to the ciliary function of IFT20, we knocked down another ciliary protein, Kif3A, which affects cilium formation as we and others recently reported9,41. We induced the expression of a dominant negative Kif3A (dnKif3A) in mature DGCs via co-injection of AAV9-CaMKII-Cre and AAV9-DIO-dnKif3A-dTomato into the dentate gyrus of wild type (WT) mice. Similar to what we observed in IFT20(−/−)mDGCs mice, we found that mice expressing dnKif3A in mature DGCs exhibited increased LTP above levels observed in WT (117.75 ± 8.25% of baseline for the WT and 163.69 ± 18.59% of baseline for the dnKif3A; p = 0.048; n = 5,4; Fig. 4d,e). Together, this set of results suggests a potential role of primary cilia in synaptic plasticity at the MF pathway of mature DGCs.
Discussion
In this study, we deleted IFT20 or disrupted the function of Kif3A to impair ciliary stability42,43 of mature DGCs to decipher the function of primary cilia in hippocampal physiology. Conditionally knocking out IFT20 in mature DGCs, we observed defects in contextual fear memory and pre-exposure contextual fear memory expression, as evidenced by decreased freezing behavior upon exposure to a previously encountered fear context. Importantly, anxiety-like behaviors were unaffected after cilia depletion, demonstrating that differences in basal anxiety-like states are unlikely to account for the observed differences in contextual fear memory expression. We also observed a deficit in spatial recognition memory, as evidenced by a lack of preferential exploration of a novel object location in IFT20(−/−)mDGCs mice. On the other hand, contextual pattern separation abilities were spared, implicating primary cilia as important in some, but not all forms of hippocampus-dependent contextual memory. Finally, we discovered a substantial abnormality in the plasticity of MF synapses after cilia depletion, which may account for the behavioral impairments observed. In sum, our data suggest a function of mature DGCs’ primary cilia in hippocampus-dependent behavior and plasticity.
Though the major downstream signaling pathways of primary cilia that lead to the observed disruptions remain to be determined, the Wnt/β-catenin pathway is a possible candidate, given previous findings by others and us9,44. Moreover, primary cilia are enriched with several G-protein couple receptors including somatostatin receptor 3 (SSTR3), serotonin receptor subtype 6 (5-HT6) and enzymes including ACIII, and signaling pathways downstream of these could be another possible cause. Indeed, in SSTR3 or ACIII-knockout animals, several hippocampus-dependent behavioral defects have been identified13,14. However, given that these tests have used animals with constitutively knocked out genes, it has been challenging to conclude whether these defects were the result of disruptions during development. Moreover, because both manipulations caused ciliary loss, it remains unknown whether these defects resulted from the specific loss of SSTR3 or ACIII, or from the loss of primary cilia per se. Likewise, more work will be needed to definitively confirm that the behavioral effects observed in the current study are not the result of non-ciliary functions of the proteins we disrupted.
Learning and memory generally occur via a three-step process: encoding, consolidation, and retrieval. The DG-CA3 juncture has been found to be heavily involved in the formation of a contextual memory representation29,45, which is consistent with our finding after cilium depletion in mature DGCs. Our observation is further strengthened upon finding a defect in plasticity induced in area CA3. Previously, it was reported that functional ablation of SSTR3 resulted in aberrant LTP at the Schaffer collateral synapse14. Here, we instead tested LTP in the target area likely responsible for contextual memory expression and found a substantial increase in LTP amplitude. Given the unchanged basal transmission, we assume that this likely resulted from altered plasticity factors such as NMDA receptor subunit composition or expression level7, however this requires further investigation. In any case, synaptic plasticity has been considered a cellular mechanism of memory encoding46 and memory recall during pattern completion47,48,49. After cilium depletion from mature DGCs, we found augmented plasticity in CA3 synapses. Although we are uncertain whether potentiated plasticity directly caused memory defects, given the role of the tri-synaptic circuit in memory50, the altered plasticity can at least partially explain the memory deficits. However, a novel approach to downgrade the enhanced plasticity will be helpful to address this confusion.
Our finding that depletion of IFT20 in mature DGCs leads to impairments in both CFC and PECFC suggest that primary cilia are important for proper rapid memory encoding. A potential model consistent with these findings is that primary cilia in mature DGCs may regulate the activity of mature DGCs and their CA3 output51, facilitating the rapid encoding of incoming contextual information. Furthermore, it is possible that primary cilia in mature DGCs indirectly modulate the CA3 local circuit activity to influence memory encoding. Both possibilities will require further exploration.
Finally, one potential limitation that we should point out is the viral vector-based approach for targeting a specific cell population, although this approach is still currently widely used in opto- and chemo-genetic gene delivery. In this study, we expressed Cre recombinase under the CaMKII promoter to target DGCs. Although we observed robust targeting of CaMKII-driven Cre expression to DGCs and noted no overlap between PV+ interneurons and CaMKII-driven GFP expression in the dentate gyrus, others have reported limited specificity of this promoter to excitatory neurons in cortical layer V and hippocampal area CA1, labeling some inhibitory PV+ interneurons as well52,53. In our study, we did identify a small amount of Cre expression in the hilus, thus we cannot entirely exclude a potential contribution of a small proportion of hilar mossy cells to our findings. Furthermore, there may have been a small population of interneurons also labeled. However, we conclude that the expression of Cre must have been very low since we detected no fluorescent protein nor ciliary defect. Nevertheless, more specific promoters and MiniPromoters should be explored to facilitate virus-based genetic manipulations, which will be a great complementary technology to the transgenic approach.
In the adult brain, new DGCs are continuously generated23,24,25. Several studies have demonstrated that young, immature neurons play distinct roles from those of mature neurons18,28,31,54. Manipulating mature DGCs through their primary cilia may disrupt the balance of activity between young and mature DGCs in the dentate gyrus by decreasing the neuronal activity of mature DGCs specifically. Since young DGCs are important for pattern separation31 and we observed no impairments on such a behavioral assay, our findings are consistent with the idea that mature, but not immature DGC activity, is disrupted upon cilia depletion, however further work will be required to confirm this idea. A potential increase in activity and plasticity of young neurons relative to mature neurons could explain our finding of enhanced MF LTP in IFT20(−/−)mDGCs and dnKif3A-expressing mice.
In summary, we demonstrated a physiological function of the neuronal primary cilium. Our findings implicate primary cilia of mature DGCs in hippocampus-associated contextual memory. It will be interesting to further test the cellular mechanisms by which primary cilia regulate the activity of the dentate granule neurons, and also to examine possible effects of primary cilia in other brain regions.
Additional Information
How to cite this article: Rhee, S. et al. Depletion of primary cilia from mature dentate granule cells impairs hippocampus-dependent contextual memory. Sci. Rep. 6, 34370; doi: 10.1038/srep34370 (2016).
Supplementary Material
Acknowledgments
We thank Drs David Talmage, Ken Takemaru and Amar Sahay for comments during the thesis committee meetings. We thank the critical comments from other laboratory members. The research was supported by NIH (NS065915, NS089770, and AG046875) and Feldstein Medical Foundation to S.G, and NIH (MH110103) to G.W.K.
Footnotes
Author Contributions S.R. and S.G. designed the experiments. S.R. performed the behavioral experiments. S.R. and Y.G. performed the electrophysiological experiments. S.R. and G.W.K. performed the surgeries and immunohistochemistry experiments. All authors analyzed and interpreted the data. S.R., G.W.K. and S.G. wrote the manuscript. All authors read and edited the manuscript.
References
- Satir P. & Christensen S. T. Overview of structure and function of mammalian cilia. Annual review of physiology 69, 377–400, 10.1146/annurev.physiol.69.040705.141236 (2007). [DOI] [PubMed] [Google Scholar]
- Lee J. E. & Gleeson J. G. Cilia in the nervous system: linking cilia function and neurodevelopmental disorders. Current opinion in neurology 24, 98–105, 10.1097/WCO.0b013e3283444d05 (2011). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gerdes J. M., Davis E. E. & Katsanis N. The vertebrate primary cilium in development, homeostasis, and disease. Cell 137, 32–45, 10.1016/j.cell.2009.03.023 (2009). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Han Y. G. et al. Hedgehog signaling and primary cilia are required for the formation of adult neural stem cells. Nat Neurosci 11, 277–284, 10.1038/nn2059 (2008). [DOI] [PubMed] [Google Scholar]
- Amador-Arjona A. et al. Primary cilia regulate proliferation of amplifying progenitors in adult hippocampus: implications for learning and memory. The Journal of neuroscience: the official journal of the Society for Neuroscience 31, 9933–9944, 10.1523/JNEUROSCI.1062-11.2011 (2011). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Breunig J. J. et al. Primary cilia regulate hippocampal neurogenesis by mediating sonic hedgehog signaling. Proc Natl Acad Sci USA 105, 13127–13132, 10.1073/pnas.0804558105 (2008). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tashiro A., Sandler V. M., Toni N., Zhao C. & Gage F. H. NMDA-receptor-mediated, cell-specific integration of new neurons in adult dentate gyrus. Nature 442, 929–933, 10.1038/nature05028 (2006). [DOI] [PubMed] [Google Scholar]
- Laplagne D. A. et al. Functional convergence of neurons generated in the developing and adult hippocampus. PLoS biology 4, e409, 10.1371/journal.pbio.0040409 (2006). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kumamoto N. et al. A role for primary cilia in glutamatergic synaptic integration of adult-born neurons. Nat Neurosci 15, 399–405, S391, 10.1038/nn.3042 (2012). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Badano J. L., Mitsuma N., Beales P. L. & Katsanis N. The ciliopathies: an emerging class of human genetic disorders. Annual review of genomics and human genetics 7, 125–148, 10.1146/annurev.genom.7.080505.115610 (2006). [DOI] [PubMed] [Google Scholar]
- Valente E. M., Rosti R. O., Gibbs E. & Gleeson J. G. Primary cilia in neurodevelopmental disorders. Nature reviews. Neurology 10, 27–36, 10.1038/nrneurol.2013.247 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goetz S. C. & Anderson K. V. The primary cilium: a signalling centre during vertebrate development. Nature reviews. Genetics 11, 331–344, 10.1038/nrg2774 (2010). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang Z., Phan T. & Storm D. R. The type 3 adenylyl cyclase is required for novel object learning and extinction of contextual memory: role of cAMP signaling in primary cilia. The Journal of neuroscience: the official journal of the Society for Neuroscience 31, 5557–5561, 10.1523/JNEUROSCI.6561-10.2011 (2011). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Einstein E. B. et al. Somatostatin signaling in neuronal cilia is critical for object recognition memory. The Journal of neuroscience: the official journal of the Society for Neuroscience 30, 4306–4314, 10.1523/JNEUROSCI.5295-09.2010 (2010). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Follit J. A., Tuft R. A., Fogarty K. E. & Pazour G. J. The intraflagellar transport protein IFT20 is associated with the Golgi complex and is required for cilia assembly. Molecular biology of the cell 17, 3781–3792, 10.1091/mbc.E06-02-0133 (2006). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gu Y. et al. Optical controlling reveals time-dependent roles for adult-born dentate granule cells. Nat Neurosci 15, 1700–1706, 10.1038/nn.3260 (2012). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nakashiba T., Young J. Z., McHugh T. J., Buhl D. L. & Tonegawa S. Transgenic inhibition of synaptic transmission reveals role of CA3 output in hippocampal learning. Science 319, 1260–1264, 10.1126/science.1151120 (2008). [DOI] [PubMed] [Google Scholar]
- Sahay A. et al. Increasing adult hippocampal neurogenesis is sufficient to improve pattern separation. Nature 472, 466–470, 10.1038/nature09817 (2011). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dere E., Huston J. P. & De Souza Silva M. A. Episodic-like memory in mice: simultaneous assessment of object, place and temporal order memory. Brain Res Brain Res Protoc 16, 10–19, 10.1016/j.brainresprot.2005.08.001 (2005). [DOI] [PubMed] [Google Scholar]
- Komada M., Takao K. & Miyakawa T. Elevated plus maze for mice. J Vis Exp, 10.3791/1088 (2008). [DOI] [PMC free article] [PubMed] [Google Scholar]
- van Praag H. et al. Functional neurogenesis in the adult hippocampus. Nature 415, 1030–1034, 10.1038/4151030a (2002). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jonassen J. A., San Agustin J., Follit J. A. & Pazour G. J. Deletion of IFT20 in the mouse kidney causes misorientation of the mitotic spindle and cystic kidney disease. The Journal of cell biology 183, 377–384, 10.1083/jcb.200808137 (2008). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ming G.-l. & Song H. Adult Neurogenesis in the Mammalian Central Nervous System. 10.1146/ (2005). [DOI] [PubMed]
- Gage F. H. Mammalian neural stem cells. Science 287, 1433–1438 (2000). [DOI] [PubMed] [Google Scholar]
- Bonaguidi M. A. et al. In vivo clonal analysis reveals self-renewing and multipotent adult neural stem cell characteristics. Cell 145, 1142–1155, 10.1016/j.cell.2011.05.024 (2011). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dittgen T. et al. Lentivirus-based genetic manipulations of cortical neurons and their optical and electrophysiological monitoring in vivo. Proc Natl Acad Sci USA 101, 18206–18211, 10.1073/pnas.0407976101 (2004). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gleeson J. G., Lin P. T., Flanagan L. A. & Walsh C. A. Doublecortin is a microtubule-associated protein and is expressed widely by migrating neurons. Neuron 23, 257–271 (1999). [DOI] [PubMed] [Google Scholar]
- Nakashiba T. et al. Young dentate granule cells mediate pattern separation, whereas old granule cells facilitate pattern completion. Cell 149, 188–201, 10.1016/j.cell.2012.01.046 (2012). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Leutgeb J. K., Leutgeb S., Moser M. B. & Moser E. I. Pattern separation in the dentate gyrus and CA3 of the hippocampus. Science 315, 961–966, 10.1126/science.1135801 (2007). [DOI] [PubMed] [Google Scholar]
- Leutgeb S. & Leutgeb J. K. Pattern separation, pattern completion, and new neuronal codes within a continuous CA3 map. Learning & memory 14, 745–757, 10.1101/lm.703907 (2007). [DOI] [PubMed] [Google Scholar]
- Sahay A., Wilson D. A. & Hen R. Pattern separation: a common function for new neurons in hippocampus and olfactory bulb. Neuron 70, 582–588, 10.1016/j.neuron.2011.05.012 (2011). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Olton D. S. & Papas B. C. Spatial memory and hippocampal function. Neuropsychologia 17, 669–682 (1979). [DOI] [PubMed] [Google Scholar]
- Drew M. R. & Hen R. Adult hippocampal neurogenesis as target for the treatment of depression. CNS Neurol Disord Drug Targets 6, 205–218 (2007). [DOI] [PubMed] [Google Scholar]
- Sahay A., Drew M. R. & Hen R. Dentate gyrus neurogenesis and depression. Prog Brain Res 163, 697–722, 10.1016/S0079-6123(07)63038-6 (2007). [DOI] [PubMed] [Google Scholar]
- Santarelli L. et al. Requirement of hippocampal neurogenesis for the behavioral effects of antidepressants. Science 301, 805–809, 10.1126/science.1083328 (2003). [DOI] [PubMed] [Google Scholar]
- Kheirbek M. A., Klemenhagen K. C., Sahay A. & Hen R. Neurogenesis and generalization: a new approach to stratify and treat anxiety disorders. Nature neuroscience 15, 1613–1620, 10.1038/nn.3262 (2012). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Snyder J. S., Kee N. & Wojtowicz J. M. Effects of adult neurogenesis on synaptic plasticity in the rat dentate gyrus. J Neurophysiol 85, 2423–2431 (2001). [DOI] [PubMed] [Google Scholar]
- Sedmak T. & Wolfrum U. Intraflagellar transport molecules in ciliary and nonciliary cells of the retina. The Journal of cell biology 189, 171–186, 10.1083/jcb.200911095 (2010). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Keady B. T., Le Y. Z. & Pazour G. J. IFT20 is required for opsin trafficking and photoreceptor outer segment development. Molecular biology of the cell 22, 921–930, 10.1091/mbc.E10-09-0792 (2011). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Finetti F. et al. Intraflagellar transport is required for polarized recycling of the TCR/CD3 complex to the immune synapse. Nat Cell Biol 11, 1332–1339, 10.1038/ncb1977 (2009). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gray R. S. et al. The planar cell polarity effector Fuz is essential for targeted membrane trafficking, ciliogenesis and mouse embryonic development. Nature cell biology 11, 1225–1232, 10.1038/ncb1966 (2009). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ishikawa H. & Marshall W. F. Ciliogenesis: building the cell’s antenna. Nature reviews. Molecular cell biology 12, 222–234, 10.1038/nrm3085 (2011). [DOI] [PubMed] [Google Scholar]
- Pedersen L. B. & Rosenbaum J. L. Intraflagellar transport (IFT) role in ciliary assembly, resorption and signalling. Current topics in developmental biology 85, 23–61, 10.1016/S0070-2153(08)00802-8 (2008). [DOI] [PubMed] [Google Scholar]
- Kuwabara T. et al. Wnt-mediated activation of NeuroD1 and retro-elements during adult neurogenesis. Nature neuroscience 12, 1097–1105, 10.1038/nn.2360 (2009). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Daumas S., Halley H., Frances B. & Lassalle J. M. Encoding, consolidation, and retrieval of contextual memory: differential involvement of dorsal CA3 and CA1 hippocampal subregions. Learning & memory 12, 375–382, 10.1101/lm.81905 (2005). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee I. & Kesner R. P. Encoding versus retrieval of spatial memory: double dissociation between the dentate gyrus and the perforant path inputs into CA3 in the dorsal hippocampus. Hippocampus 14, 66–76, 10.1002/hipo.10167 (2004). [DOI] [PubMed] [Google Scholar]
- Gold A. E. & Kesner R. P. The role of the CA3 subregion of the dorsal hippocampus in spatial pattern completion in the rat. Hippocampus 15, 808–814, 10.1002/hipo.20103 (2005). [DOI] [PubMed] [Google Scholar]
- Kesner R. P. Behavioral functions of the CA3 subregion of the hippocampus. Learning & memory 14, 771–781, 10.1101/lm.688207 (2007). [DOI] [PubMed] [Google Scholar]
- Nakazawa K. et al. Requirement for hippocampal CA3 NMDA receptors in associative memory recall. Science 297, 211–218, 10.1126/science.1071795 (2002). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu X. et al. Optogenetic stimulation of a hippocampal engram activates fear memory recall. Nature 484, 381–385, 10.1038/nature11028 (2012). [DOI] [PMC free article] [PubMed] [Google Scholar]
- DeCaen P. G., Delling M., Vien T. N. & Clapham D. E. Direct recording and molecular identification of the calcium channel of primary cilia. Nature 504, 315–318, 10.1038/nature12832 (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nathanson J. L., Yanagawa Y., Obata K. & Callaway E. M. Preferential labeling of inhibitory and excitatory cortical neurons by endogenous tropism of adeno-associated virus and lentivirus vectors. Neuroscience 161, 441–450, 10.1016/j.neuroscience.2009.03.032 (2009). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schoenenberger P., O’Neill J. & Csicsvari J. Activity-dependent plasticity of hippocampal place maps. Nat Commun 7, 11824, 10.1038/ncomms11824 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Clelland C. D. et al. A functional role for adult hippocampal neurogenesis in spatial pattern separation. Science 325, 210–213, 10.1126/science.1173215 (2009). [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.