Abstract
Background
Orthostatic tremor (OT) remains among the most intriguing and poorly understood of movement disorders. Compared to Parkinson’s disease or even essential tremor, there are very few articles addressing more basic science issues. In this review, we will discuss the findings of main case series on OT, including data on etiology, pathophysiology, diagnostic approach, treatment strategies, and outcome.
Methods
Data for this review were identified by searching PUBMED (January 1966 to August 2016) for the terms “orthostatic tremor” or “shaky leg syndrome,” which yielded 219 entries. We did not exclude papers on the basis of language, country, or publication date. The electronic database searches were supplemented by articles in the authors’ files that pertained to this topic.
Results
Owing to its rarity, the current understanding of OT is limited and is mostly based on small case series or case reports. Despite this, a growing body of evidence indicates that OT might be a progressive condition that is clinically heterogeneous (primary vs. secondary cases) with a broader spectrum of clinical features, mainly cerebellar signs, and possible cognitive impairment and personality disturbances. Along with this, advanced neuroimaging techniques are now demonstrating distinct anatomical and functional changes, some of which are consistent with neuronal loss.
Discussion
OT might be a family of diseases, unified by the presence of leg tremor, but further characterized by etiological and clinical heterogeneity. More work is needed to understand the pathogenesis of this condition.
Keywords: Orthostatic tremor, clinical, pathophysiology, treatment, review
Introduction
The term “orthostatic tremor” (OT), also known as “shaky legs syndrome”1 was first coined in 1984 by Heilman,2 although the earlier descriptions of this entity date back to 1970 when Pazzaglia et al.3 reported on three patients with a peculiar disorder only occurring on standing.
This is an intriguing and rare condition, characterized by unsteadiness when standing that is relieved when sitting or walking; it is confirmed on neurophysiological recording by the presence of a fast tremor of 13–18 Hz in the legs, trunk, and, sometimes, the arms, which is coherent in all muscles studied.4 OT can be idiopathic or secondary.5–7 Gerschlager et al.5 suggested the subdivision of OT into two broad groups—those with primary (idiopathic) OT with or without postural arm tremor, and those with “OT plus,” in whom there are additional associated movement disorders, mainly parkinsonism.
Compared to Parkinson’s disease or even essential tremor, there are very few articles addressing more basic science issues (e.g. laboratory studies included gene discovery, molecular biology, and cellular biology) and neuroimaging studies. Scientific understanding of OT is in its infancy. In this review, we will discuss the findings of series on OT, including data on etiology, diagnostic approach, treatment strategies, and outcome of this challenging condition.
Methods
Data for this review were identified by searching PUBMED (January 1966-August 2016) for the terms “orthostatic tremor” or “shaky leg syndrome,” which yielded 219 entries. We did not exclude papers on the basis of language, country, or publication date. The electronic database searches were supplemented by articles in the authors’ files pertaining to this topic.
Epidemiology
Prevalence, incidence, and mortality rates
As there are no published population-based epidemiological data, the prevalence and incidence of OT are unknown; however, it is considered to be a very rare entity. In the Neurological Disorders of Central Spain (NEDICES) study,8 our group detected one OT patient in a cohort of approximately 4,000 elderly subjects (data not published).
Mortality in OT has not yet been studied, although with the presence in some patients of functional disability,6,9 gait involvement, and higher likelihood of falls,10 mainly in those with lower-frequency tremor discharges,11 and possible cognitive impairment,12 it is conceivable that OT might influence the risk of mortality.
Risk and etiological factors
Although most patients first develop OT around 60 years of age, the age range at onset is wide, from 13 to 85 years.5,9,10,13 Adolescent or pediatric OT cases have rarely been reported.10 Hence, given the few cases published in the literature, it is not possible to compare the typical (e.g. late onset) vs. early onset courses of disease. Age at onset may differ depending on whether OT is primary or associated with other neurologic features.5 In one classic clinical case series including 41 subjects, age at onset was significantly earlier in primary OT (50.4±15.1 years ) than in the OT with associated neurologic features group (61.8±6.4 years, p = 0.006).5 A review of published case series indicates that OT is a disorder predominantly affecting female seniors.5,9,10,13
The vast majority of cases of OT appear to be sporadic; however, a few examples of familial cases in monozygotic twins,14 siblings,15–17 or in a mother and her son13 have been published. In the largest clinical series of the literature (184 patients), Hassan et al.,10 reported that family history of OT was noted in 4.9%. Furthermore, there may be a family history of Parkinson’s disease or other types of tremor.13 In a recent series of 45 OT patients, a family history of any tremor was noted in 51% patients.18 Of interest, a family history of OT was reported in 7% patients.18
Molecular defects have not been systematically studied in OT. There is a single study that has identified a C10orf2 Twinkle mutation in a man with OT in his late sixties and progressive external ophthalmoplegia.19 Twinkle is a nuclear-encoded human mitochondrial DNA helicase.19 This observation raises the question of a possible role of mitochondrial dysfunction in the genesis of OT.19 There is another report of a 63-year-old female with OT carrying a REEP1 mutation (formerly SPG31), which is almost exclusively associated with a pure hereditary spastic paraparesis phenotype.20 The key question remains whether the OT seen here is part of SPG31 or is coincidental.20 Given that these two disorders are very rare, their co-occurrence by chance would be very unlikely.20
Most cases of OT are idiopathic, with normal brain neuroimaging, normal laboratory work-up, and no evidence of other associated conditions.10 There are however some rare examples of structural causes of OT or associated conditions after an extensive laboratory and neuroimaging work-up (Table 1). Although all of these patients presented with clinical features for OT (unsteadiness when standing that is relieved when sitting or walking), a number of them did not actually have typical high-frequency electromyography (EMG) discharges (13–18 Hz) (Table 1). Secondary (symptomatic) cases have been described in patients with non-tumoral aqueduct stenosis,21 relapsing polyradiculoneuropathy,21 head trauma,22 pontine and midbrain lesions,23,24 cerebellar degeneration,25–27 spinal cord lesion,28 paraneoplastic syndrome associated with small cell lung cancer,29 stiff-person syndrome,30 multiple sclerosis,31 Graves’ disease,32–34 biclonal immunoglobulin (Ig)G and IgA lambda gammopathy of undetermined significance,35 thiamine deficiency,36 vitamin B12 deficiency,1 and secondary to recreational use of solvents.37 Of interest is the description of four patients who developed reversible OT after exposure to dopamine-blocking drugs.38
Table 1. Clinical Characteristics of Published Symptomatic Orthostatic Tremor Cases.
| Conditions | Frequency (Hz) | Other Systemic or Neurological Features | Evolution |
|---|---|---|---|
| Non-tumoral aqueduct stenosis21 | 6–7 | Bradykinesia, rigidity, and postural tremor of upper limbs | Remission with ventriculoperitoneal shunt |
| Chronic relapsing polyradiculoneuropathy21 | 6–7 | Dysesthesias and mild weakness of the limbs, and postural tremor of upper limbs | Remission with prednisone |
| Head trauma22 | 14–15 | – | No improvement |
| Pontine lesions23 | |||
| Cavernoma | 7–8 | Face and arm paresthesia | Partial recovery |
| Tuberculoma | 14–15 | Fever, headache, left predominate postural tremor of the arms | Complete remission |
| Midbrain lesion24 | 16 | Truncal and cranial muscles involvement | Partial recovery |
| Cerebellar degeneration25 | 14–15 | Scanning speech, intention tremor, ataxic gait | – |
| Cerebellar degeneration26 | Gaze-evoked nystagmus, dysmetria of saccades, scanning speech, intention tremor in 4 limbs, and broad-based ataxic gait | ||
| Patient 1 | 14 | Slightly improvement with clonazepam | |
| Patient 2 | 15 | No improvement | |
| Patient 3 | 14 | No improvement | |
| Cerebellar degeneration27 | |||
| Patient 1 | 13.8–15.9 | Dysmetria, ataxic gait, cognitive impairment, and nystagmus | No improvement |
| Spinocerebellar ataxia type 2 | 13 | Dysarthria, dysmetria, ataxic gait, cognitive impairment, and slowing of horizontal pursuit | Improvement with propranolol |
| Spinal cord lesion,28 | 12–13 | Cognitive impairment, voiding difficulty, and postural instability | Complete remission after spinal schwannoma |
| Small cell lung cancer29 | 3–5 | Head and arms tremor, persistence during walking | Rapid worsening |
| Stiff-person syndrome30 | |||
| Patient 1 | – | Stiffness and spasms | Improvement with |
| Patient 2 | 16 | Stiffness and spasms | clonazepam |
| Multiple sclerosis31 | 4 | Dysmetria, ataxia, pyramidal signs and sensorial deficits | No improvement |
| Graves’ disease32 | 14–16 | – | Complete remission after carbimazole |
| Graves’ disease33 | 8-9 | – | Complete remission after methimazole |
| Graves’ disease34 | 8 | Intolerance of heat, excessive sweating and recent loss of weight with increased appetite, dysphagia and dysphonia | Complete remission after methimazole |
| Biclonal IgG and IgA lambda gammopathy of undetermined significance,35 | 17.5 | – | Improvement with gabapentin |
| Thiamine deficiency36 | – | Diplopia, gait difficulty | Slow improvement |
| Vitamin B12 deficiency1 | 15 | Impaired sensation of pain and heat in a stocking distribution | Complete remission after vitamin B12 and clonazepam |
| Secondary to recreational use of solvents.37 | 15 | – | Improvement with clonazepam |
| Secondary to dopamine blocking drugs38 | |||
| Metoclopramide | 5–6 | Mild rigidity | Improvement |
| Sulpiride and thyethylperazine | 6–7 | Resting tremor | Complete remission |
| Sulpiride | 15 | – | Complete remission |
| Sulpiride | 14 | Vertigo and nystagmus | Complete remission |
Phenomenology and clinical features
General characteristics
OT patients usually report a feeling of unsteadiness during stance without problems when sitting and lying.4 However, they rarely report tremor sensation or leg pain as a presenting symptom.5,13 Many patients try to reduce the feeling of unsteadiness by standing with a widened stance and clawing the floor with their toes.39 The onset and cessation of the symptoms depend on the severity of the disease. On occasion, it may start suddenly with position changes, from sitting to standing and vice versa.13 Other patients with milder OT may have to stand still for several minutes in order for the symptoms to appear.5,13,40 It is characteristic that the symptoms of OT decrease markedly on sitting, walking, or when leaning against a wall.5,13,40 The need to sit down or to walk can be so disturbing that patients express that they tend to avoid situations in which they have to stand still for a long period, such as taking a shower, waiting in line, or standing at a kitchen counter to prepare a meal.5,13,40 In fact, in these situations, the patients usually try to alternate weight from one leg to the other, walk in place, or lean on an object such as a chair or a countertop.40 In more advanced stages, patients may demonstrate abnormalities in tandem gait; these abnormalities are indistinguishable from those seen in patients with cerebellar diseases.40
Progression of OT has been described. In most of the OT patients followed by Gerschlager et al.,5 the symptom severity was relatively unchanged over time, but in six of their 41 patients there was a clear progression of symptom severity with time, as measured by the amount of time they could stand still. In four of these, a clear spread of tremor was confirmed, with tremor initially only involving the leg muscles and then spreading proximally to involve the trunk and arm muscles.5 By contrast, in a recent series of 68 OT patients, a total 79.4% of patients reported worsening of their OT tremor symptoms.9 These patients had significantly longer symptom duration than those without reported worsening (median 15.5 vs. 10.5 years, respectively; p = 0.005).9 However, there was no change in OT frequency over time.9 In another recent series, in 11 out of 30 cases, OT was reported to be worse over a 54.4-month follow-up period.18 However, in the previous studies,5,9,18 the progression of symptoms had been described by clinical impression and information from the patients. Feil et al.,41 examined 15 patients with primary OT from a clinical cohort over time using serial posturographic measurements.41 Posturographic data (5.4±4.0 years) revealed a significant increase in the total sway path (standing on firm ground with eyes open) from 2.4±1.3 to 3.4±1.4 meter/minute (p = 0.022) and of the total root mean square values from 9.8±4.3 to 12.4±4.8 mm (p = 0.028).41 Using objective measurements, this long-term follow-up study indicates that primary OT is a progressive disorder.41
Apart from in the legs, tremor is often present in other areas such as the hands, cranial muscles, and even the trunk.13,42 In fact, only a small proportion of patients have isolated leg tremors.13 Patients with primary (idiopathic) OT may be divided into those with vs. those without a postural arm tremor.5 The postural tremor is similar to that observed in essential tremor patients.5 Indeed, the tremor becomes obvious when the patient maintains their arms outstretched against gravity in front of their body (e.g. extending the upper limbs horizontally) and typically has a frequency of 5–10 Hz, which overlaps with the frequency seen in essential tremor patients.13 Most OT patients have such postural tremor, with the proportion ranging from 77.4% to 92.3%.5,13,18
There have been a few reports of patients whose OT was associated with other movement disorders, mainly parkinsonism and specifically Parkinson’s disease.5,6 Of the 41 patients included in the clinical series by Gerschlager et al.,5 other additional neurological features were evident in 10. Specifically, six had parkinsonism (four had typical Parkinson’s disease, one vascular parkinsonism and restless leg syndrome, and one had drug-induced parkinsonism). Of the remaining four patients, two also had restless leg syndrome, one had tardive dyskinesia of uncertain etiology, and one had orofacial dyskinesias of uncertain etiology.5 In another clinical series, the associated movement disorders were Parkinson’s disease (n = 1), parkinsonism (n = 1), progressive supranuclear palsy (n = 1), restless leg syndrome (n = 1), multifocal action tremor (n = 2), pathological proven dementia with Lewy bodies (n = 1), and focal dystonia of the arm (n = 1).6 Further, a few OT patients may develop incident Parkinson’s disease 5,9,43 or progressive supranuclear palsy months or years later.44 Similarly, OT may appear in long-standing Parkinson’s disease after 10 years.45,46
Clinical examination
From the first descriptions, OT has been reported to appear on standing, is associated with a profound and disabling sense of unsteadiness, and is relieved by sitting, walking or the use of a support.2,5 It is generally accepted that classic OT manifests with a high-frequency tremor (13–18 Hz), which means it may not be visible on routine examination,5,13,40 and this may make the diagnosis challenging. When patients complain that they feel unsteady on their feet, clinicians may overlook the possibility of OT, and may focus on other causes of unsteadiness. The examination reveals a rapid tremor of the legs on standing, which may sometimes be palpable as a fine-amplitude rippling of leg muscles (e.g. the gastrocnemius or quadriceps muscles) with an associated knee tremor; the tremor may be more easily felt than seen because of its high frequency.47 Auscultation using a stethoscope of the gastrocnemius muscles can sometimes reveal a characteristic of barely audible noise akin to the sound of distant rotor blades of a helicopter.48
Neurophysiologic characteristics
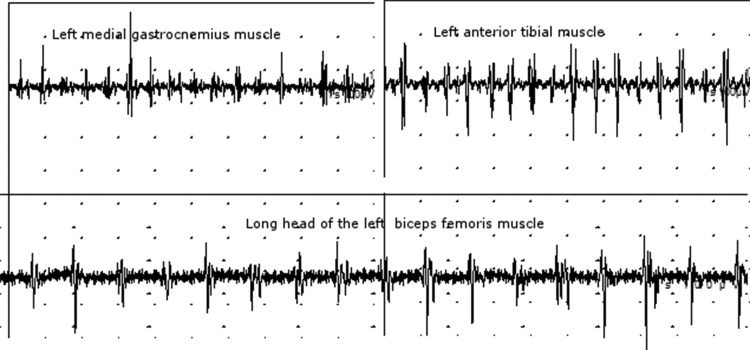
The diagnosis of OT is based on history and physical examination.4 However, the diagnosis should be confirmed by surface EMG recordings revealing on standing rhythmic activation of lower limb muscles at sharply peaked frequencies between 13 and 18 Hz, and sometimes higher (Figure 1).4,39 This rhythmic activity disappears when the patient is seated or lifted off the ground. Another feature is that these brief, highly rhythmic discharges demonstrate high intermuscular coherence.39 These singular neurophysiologic characteristics are part of the criteria established by the Movement Disorders Society (Table 2).4
Figure 1. Typical high-frequency electromyography discharges in orthostatic tremor. Surface electromyography recordings of a 57-year-old female orthostatic tremor patient from left leg muscles showing discharges at the frequency of 16–18 Hz with the patient standing.
Table 2. Consensus Statement on Tremor by the Movement Disorder Society. Criteria for Orthostatic Tremor.
| Orthostatic tremor is a unique tremor syndrome characterized by: |
| 1. A subjective feeling of unsteadiness during stance but only in severe cases during gait; patients rarely fall. None of the patients have problems when sitting and lying. |
| 2. Sparse clinical findings that are mostly limited to a visible and occasionally, only palpable fine amplitude rippling of the leg (quadriceps or gastrocnemius) muscles when standing. |
| 3. The diagnosis that can be confirmed only by electromyography recordings (e.g. from the quadriceps muscle) with a typical 13–18 Hz pattern. All of the leg, trunk, and even arm muscles can show this tremor, which is typically absent during tonic activation while the patient is sitting and lying. |
Arriving at the correct diagnosis is dependent on the medical history and detailed clinical and EMG investigations. The definition of OT states that the tremor frequency should be confirmed by EMG; however, in practice, an accelerometer is an acceptable alternative in cases with typical symptoms.39 Further, an electrocardiogram recorded in the standing position could also be a simple non-invasive tool to screen for or to support the clinical diagnosis of OT. In this sense, Littmann49 reported a patient with OT in whom telemetry strips while standing revealed continuous gross 13–18 Hz of oscillatory artifact, identical to the frequency range of oscillations recorded with EMG recordings from the thigh muscles in OT patients.
Laboratory and neuroimaging work-up
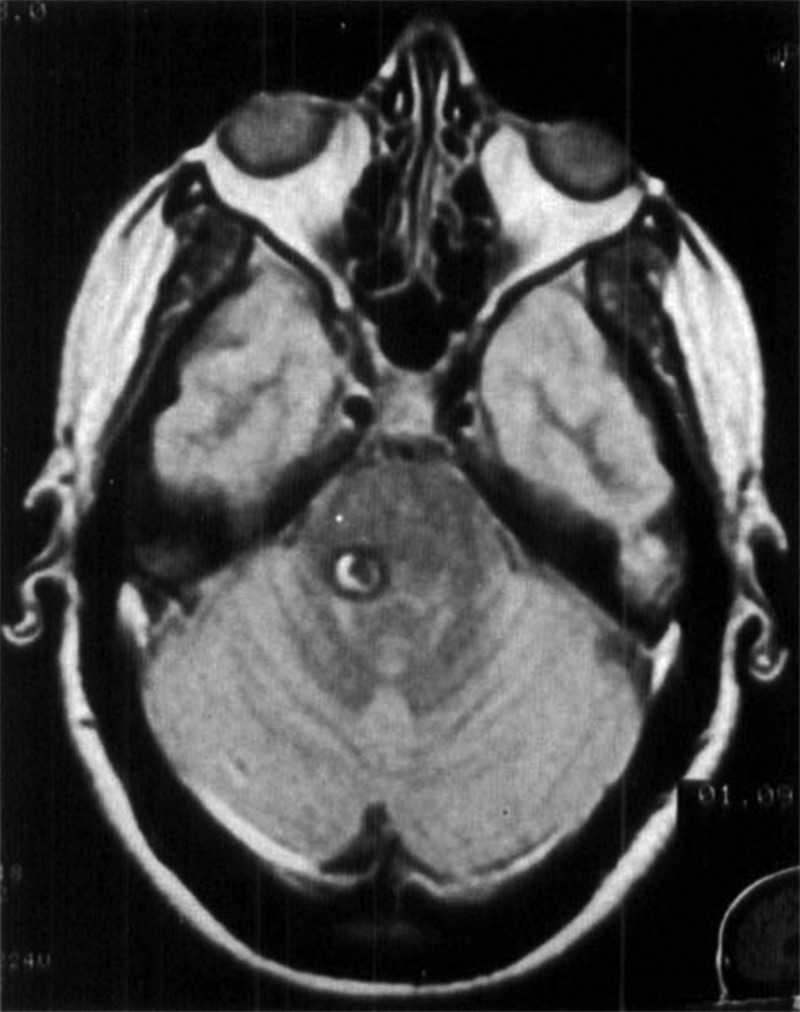
Currently, there are no laboratory findings that are typical of OT. In fact, it is considered to be an idiopathic disorder, as studies with cerebral neuroimaging and other examinations are generally normal. Hence, the purpose of laboratory investigations is to help exclude other disorders or possible symptomatic (secondary) cases. In specific patients, screening investigations should include thyroid function tests, serum protein electrophoresis to rule out gammopathies, vitamin B12 levels, diagnostic studies to exclude Wilson’s disease (e.g. serum ceruloplasmin), and dopamine transporter imaging to rule out Parkinson’s disease. Brain MRI should be performed to rule out structural causes of OT such as pontine (Figure 2) and midbrain lesions or cerebellar atrophy. In some cases with bilateral pyramidal tract signs, or with a sensory level, spinal MRI could be useful to detect spinal cord lesions.28
Figure 2. Symptomatic orthostatic tremor caused by a lesion in the posterior fossa. T1-weighted axial magnetic resonance imaging scan showing a lesion involving the right pontine region, compatible with a cavernoma. Case 1 from Benito-León J, Rodríguez J, Ortí-Pareja M, et al. Symptomatic orthostatic tremor in pontine lesions. Neurology 1997;49:1439–1441. Reprinted with permission from Wolters Kluwer Health.
Differential diagnosis
OT is not widely recognized by physicians who are not movement disorders experts, which often results in misdiagnosis for the unfortunate patients, who then may be subjected to inappropriate or unnecessary tests and treatment. In fact, it is often misdiagnosed as essential tremor, Parkinson’s disease, restless leg syndrome, lumbar stenosis, and especially non-organic (psychogenic) balance disorders.5,13,50 Of note, when Pazzaglia et al.3 saw the first of their three patients in January 1968 they were left perplexed and puzzled and doubted its true organic nature.
A lack of recognition may lead to misdiagnosis; the fact that the key physical signs are subtle and easily missed can further contribute to that misdiagnosis.5,13 Indeed, in the series by Gerschlager et al,5 5.7 years elapsed between reported symptom onset and diagnosis. In another series, the duration of symptoms prior to diagnosis of OT was 6.7 years.18 Finally, the series by Hassan et al.10 reported that diagnosis was delayed by a mean of 7.2 years.
The differential diagnosis includes a number of conditions that are characterized by unsteadiness or tremor while standing. As a similar tremor can be induced in normal persons via vestibular stimulation,51 conditions that cause imbalance should be also considered.
There is an intense debate whether OT is a distinct condition or a variant of essential tremor. Although tremor of the legs may occur in essential tremor, it always occurs with upper limb tremor, and at frequencies below 12 Hz, unlike OT.52,53 The main reason to consider the link between both diseases is that a sizable number of OT patients have a 5–10 Hz postural arm tremor,18 although only a few of them have a family history of essential tremor;13 however, those lower-frequency arm oscillations in OT may represent a subharmonic of the higher-frequency tremors typical of OT, spreading throughout the body.54 As we have commented above, the tremor of OT has two singular features. First, its high frequency (13–18 Hz) and, second, its high coherence values between homologous muscles of the two legs (e.g. the left and right gastrocnemius).39 These findings differ from those of essential tremor, in which tremor typically has a lower frequency (4–12 Hz) and in which there are lower coherence values between homologous muscles of the right and left side.39,52,53 Further, ethanol intake has little efficacy in OT patients,5,13,55 unlike essential tremor, in which the tremor improves in a large proportion of patients.52,53 Finally, in contrast to the tremor of essential tremor, OT shows little or no response to propanolol.5,13,56
Orthostatic myoclonus is a disorder that was first reported in 2007 in 15 elderly subjects.57 This condition is characterized by unsteadiness during orthostatism and/or during gait and short duration myoclonic bursts that occurred predominately with the assumption of an upright posture.57 EMG surface recording is essential for the diagnosis of this entity.57 Unlike OT, the bursts are shorter in duration, non-rhythmic, and irregular.57 Seven of these patients had a neurodegenerative disorder and two had a systemic illness known to be associated with myoclonus.57 In the remaining six patients, no known neurological disorder contributed to the phenomenon. Leu-Semenescu et al.46 also described this syndrome in three Parkinson’s disease patients complaining of unsteadiness on standing. Orthostatic myoclonus may be underdiagnosed and can be considered a possible cause of unsteadiness in subjects with neurodegenerative diseases. In a recent study involving 93 subjects who were referred to a clinical neurophysiology unit in Pamplona (Spain) for investigation of abnormal movements in orthostatism as a possible cause of their unsteadiness and/or gait impairment, from January 2007 to September 2011, orthostatic myoclonus was the most frequent disorder (17.2%), followed by OT (13.9%) and low-frequency tremors during orthostatism (12.9%).58 Eleven out of 16 patients (68.7%) with orthostatic myoclonus had an associated neurodegenerative disease, such as multiple system atrophy (n = 3) Parkinson’s disease (n = 2), Alzheimer’s disease (n = 2), mild cognitive impairment (n = 2), and normal pressure hydrocephalus (n = 2).58
In Parkinson’s disease, low-frequency (4–6 Hz) leg tremor is rarely seen while patients are standing.46,59 Overall, the response of this type of tremor to dopaminergic drugs is good.46,59 Thomas et al.60 reported four patients with a disabling standing tremor that appeared years before parkinsonian symptoms were evidenced. The tremor, whose main frequency was 6.2–6.9 Hz, with sporadic subharmonics at 8–18 Hz, was refractory to gabapentin and dramatically responded to levodopa administration.60 The authors suggested the term “pseudo-orthostatic tremor” to define this levodopa-responsive, 6–7 Hz standing tremor preceding a parkinsonian syndrome.60
What are the most significant emerging milestones that could be expanding the clinical spectrum of OT?
The three major clinical findings that could be expanding the clinical spectrum of OT are as follows.
First, from the first descriptions,61 a number of patients initially reported as clinical OT (based on the subjective feeling of unsteadiness on standing) did not actually have typical high-frequency EMG discharges (13–18 Hz).11 These patients reported as “slow OT” do have 70–120 ms EMG bursts at lower frequencies (<13 Hz).11 In slow OT, EMG coherence analysis reveals significant bilateral coupling at tremor frequency between EMG recorded from lower limb, upper limb, and axial muscles (coherence 0.2–0.8), which is absent in controls under normal conditions, and patients with orthostatic myoclonus, and is not as strong as that seen in fast OT (coherence 0.8–1).62 In addition to idiopathic cases,63,64 slow OT has been associated with familial essential tremor,61 multiple sclerosis,31 Graves’ disease,33 Parkinson’s disease and parkinsonism.65 Slow OT would seem to represent a different clinical entity from classic fast OT with more heterogeneous clinical and neurophysiologic features. It would be a summary of many conditions and presentations resembling “classical OT,” many of which present with sub-harmonic peaks of an actual “fast”/classical OT (see, for example, EMG traces of the first report of slow OT in Wee et al.,61 where the EMG in figure 2 actually exhibits a 16 Hz pattern), or non-rhythmical presentations, which are unlikely “tremor” (orthostatic myoclonus?) and would therefore speak against an inclusion into an “expanded” spectrum of OT.
Second, accumulating evidence suggests that OT is a disease of the cerebellar and/or cerebellar system. For example, in a recent clinical series of 18 OT patients, careful clinical examination revealed that at least mild cerebellar ocular motor dysfunction such as saccadic smooth pursuit was found in all the patients with most of them also showing different forms of nystagmus and with 14 out of 18 showing additional ataxia of at least one limb.41 This is in line with a recent multimodal neuroimaging study involving 17 OT patients and 17 age- and gender-matched healthy volunteers, which has demonstrated functional relevance of cerebello-thalamo-cortical connections in tremor related to cerebellar defects.66 Specifically, compared with healthy volunteers, gray matter volume in OT patients was 1) increased in the cerebellar vermis and correlated positively with the duration of the standing position; and 2) increased in the supplementary motor area and decreased in the lateral cerebellum, which both correlated with the disease duration.66 Further, functional connectivity between the lateral cerebellum and the supplementary motor area was abnormally increased in OT patients, and correlated positively with tremor severity.66 Of interest was that after repetitive transcranial stimulation, tremor severity and functional connectivity between the lateral cerebellum and the supplementary motor area were reduced.66
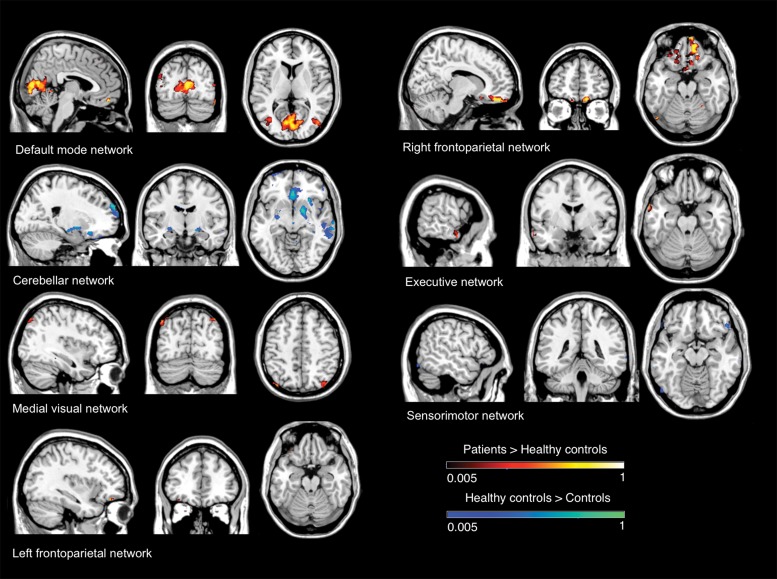
Finally, the link between OT and the cerebellum is of interest, especially in light of the role the cerebellum plays in cognition,67 and it raises the possibility that OT patients could have cognitive deficits consistent with cerebellar dysfunction. Cognitive function in OT has been the subject of a recent case–control comparison.12 Sixteen OT patients scored worse than 32 healthy matched controls on widespread cognitive domains, including deficits in executive function, visuospatial ability, verbal memory, visual memory, and language.12 Further, OT patients scored higher (i.e. greater psychopathology) on several personality assessment inventory subscales (somatic concerns, anxiety related disorders, depression, and antisocial features).12 Indeed, OT patients seem to have deficits in specific aspects of neuropsychological functioning, particularly those thought to rely on the integrity of the prefrontal cortex, which suggests involvement of frontocerebellar circuits.12 The question is whether these cognitive impairment and personality disturbances may be non-motor manifestations of OT. These results were supported by a recent functional MRI study comparing resting-state functional connectivity in OT patients and healthy controls, and specifically assessing cognitive processing networks.68 Overall, relative to healthy controls, OT patients increased connectivity in resting-state networks involved in cognitive processes (default mode network, as well as in executive and frontoparietal networks), and decreased connectivity in motor control (cerebellum and sensorimotor networks) (Figure 3).68 Changes in network integrity were associated not only with duration (default mode network and visual network), but also with cognitive function.68 Moreover, in at least two networks (default mode network and visual network), increased connectivity was associated with worse performance on different cognitive domains (attention, executive function, visuospatial ability, visual memory, and language).68 Resting-state functional MRI studies might provide a novel method to understand the pathophysiological mechanisms of motor and non-motor features of OT.
Figure 3. Resting state functional MRI findings in orthostatic tremor. Resting state brain networks showing significant functional connectivity differences between a series of 13 orthostatic tremor patients vs. 13 matched healthy controls.
Severity assessment of orthostatic tremor
There is not a validated tool to measure severity of symptoms and disability related to OT. Hence, there is a need for instruments to monitor OT clinically. What is indisputable is that OT may be a progressing and disabling condition that may negatively impact on different spheres of the health-related quality of life of those who suffer this condition, including occupational and daily living activities, as the patients tend to avoid situations where they have to stand still.39 There is nowadays increasing recognition that the global wellbeing of patients with chronic neurological diseases is an important outcome in research and clinical practice alike.69–71 Subjective (i.e. self-reported) measures of health-related quality of life may serve to alert clinicians to areas that would otherwise be overlooked.69–71 There are only two studies that have studied specifically health-related quality of life in OT.72,73 Gerschlager et al.72 applied the SF-36 and the Beck Depression Inventory to measure health-related quality of life and depression, respectively, in 20 OT patients. All dimensions of the SF-36 were markedly reduced in OT patients and depression was found in 11 out of 20 patients.72 Rodrigues et al.,73 using a modified Parkinson’s disease questionnaire (PDQ-39), observed in five OT patients that mobility, activities of daily living, bodily discomfort, emotional wellbeing, and cognition dimensions were domains that were affected in patients with OT, and these problems improved slightly with gabapentin. Overall, these two studies suggest that OT strongly impacts on health-related quality of life.
Pathophysiology
OT might be generated by a central oscillator
The traditional hypothesis about pathogenesis of OT posits the existence of a central oscillator mostly located in the brainstem and/or the cerebellum.74–76 The hypothesis that OT is generated in these areas is based on several observations. First, a small number of symptomatic OT cases have cerebellar atrophy or have lesions in the pons or midbrain.23–27 Second, one positron emission tomography study of four OT patients revealed bilateral activation of the cerebellar hemispheres as well as activation of the cerebellar vermis, thalamus, and lentiform nucleus.77 Third, posturographic data from some OT patients are indistinguishable from those seen in patients with cerebellar diseases.41 Fourth, most patients had cerebellar signs on detailed clinical examination.41 Fifth, a recent multimodal neuroimaging study has demonstrated functional relevance of cerebello-thalamo-cortical connections in tremor related to cerebellar defects.66 Sixth, cerebellar outflow (dentato-rubro-thalamic) pathways are the target of deep brain stimulation, which may be effective in treating OT.10,78–84 Finally, the tremors recorded in each leg have a high coherence. In other words, they have an almost constant phase relationship, which is not typical for most other pathological tremors.39 These findings suggest that the OTs detected in each leg originate from the same central tremor generator.85 In addition, 16 Hz EMG bursts are time-locked in the arm, leg, truncal, and even facial muscles, and are bilateral.54 Finally, the observation that unilateral transcranial magnetic stimulation of the cortical leg area resets OT in both legs, whereas OT is not modified by any peripheral stimuli, supports the hypothesis of a unique supraspinal OT generator.75 The circuitry involving in OT pathogenesis, however, may be complex, with feedback and feed forward modulation (i.e. involving several brain regions).13 There is some evidence of a potential role of the nigrostriatal dopaminergic system in the generation of OT. An association of OT with parkinsonism and treatment effects of levodopa and dopamine agonists have been reported.5,43,86,87 By means of single-photon emission computed tomography (SPECT) using 123I-FP-CIT ([123I]-2β-carbomethoxy-3β-(-4-iodophenyl)-N-(3-fluoropropyl)-nortropane) as dopamine transporter tracer, the dopaminergic system was found to be affected in a group of 11 OT patients, although to a lesser extent than in Parkinson’s disease.87 When compared with a group of 12 Parkinson’s disease patients, tracer uptake in OT patients was significantly higher and more symmetrical, and the caudates and putamens were equally affected.87 A study using transcranial sonography to examine the morphology of the substantia nigra in four OT patients showed echogenicity in all of them (unilateral in three and bilateral in one patient), suggesting the presence of nigrostriatal dopaminergic deficits.88 However, these findings are not universal, and other functional imaging studies have shown intact serotonergic and dopaminergic systems.89–91 Taken together, these findings suggest that, although the OT generator might be located in the brainstem and/or the cerebellum, other brain regions such as the motor and sensory cortices, and the basal ganglia may also be involved.76
OT may be neurodegenerative
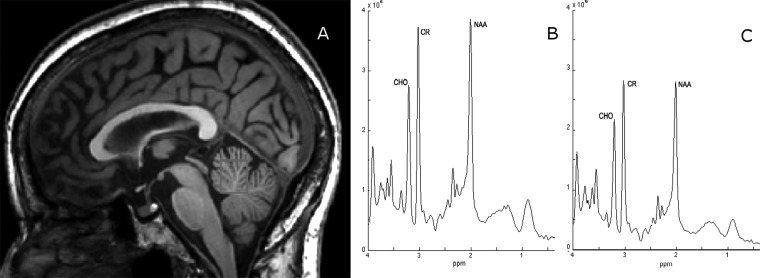
OT shares a number of important features with neurodegenerative diseases. First, OT is a progressive disorder with increasing disability, suggesting that the underlying pathological process may not be static.6,9 Second, patients who initially present with isolated OT often later develop additional neurological signs, including Parkinson’s disease.5,9,43 Third, a reduction in cerebral cortical and cerebellar cortical viability in OT has been recently published.92 Using proton magnetic resonance spectroscopy in a sample of 14 OT patients vs. 14 matched healthy controls, in mid-parietal gray matter spectra, a significant decrease in the absolute concentration of N-acetylaspartate+N-acetylaspartyl (NAA) glutamate in the patients vs. the controls (7.76±0.25 vs. 8.11±0.45, p = 0.017) was observed.92 A similar decrease in NAA was seen in the cerebellar vermis (7.33±0.61 vs. 8.55±1.54, p = 0.014) and cerebellar white matter (8.54±0.79 vs. 9.95±1.57, p = 0.010) of the cerebellum (Figure 4).92 No differences in the other metabolites or their ratios were observed.92 Fourth, as in other neurodegenerative diseases93,94 cognitive deficits, mainly in frontal executive function and memory, have been reported to occur in OT patients.12
Figure 4. T1-weighted sagittal magnetic resonance image showing the region of interest (cerebellar vermis) for the proton magnetic resonance spectroscopy study by Benito-León et al.92 The labeled resonances are those of N-acetylaspartate+N-acetylaspartyl glutamate (NAA), total creatine (CR) and total choline-containing compounds (CHO). The spectra are plotted on the same vertical intensity scale normalized to background noise. ppm, Parts Per Million. Note the reduced NAA resonance in a 61-year-old female orthostatic tremor patient (C) compared to that from a matched healthy subject (B), whereas there is a minor decrease in either CHO or CR.
The conceptualization of OT as a neurodegenerative condition has some clinical implications. It indicates that there is a cellular and molecular pathophysiology of OT and the disease is not merely the result of an electrical disarrangement. Another implication is that, moving forward, this makes the identification of modifiable risk factors more important, along with strategies aimed at disease prevention. In addition, future drug development could target important molecular and cellular events in the degenerative cascade.
Treatment
General considerations
As with other chronic diseases, physicians should pay attention to the psychological and social impact of illness on OT patients. Further, the impact of the disease on the patient’s family should also be considered. It may be beneficial for OT patients to bring their spouse or partner to a consultation, to help them better understand the disease and to discuss their difficulties and concerns.
Patient-centered associations may be of help in offering individual and group support, education and advice (http://www.orthostatictremor.org/). Through such interactions, patients may benefit by learning ways to cope with the many practical day-to-day difficulties that arise for those living with this disease (http://www.orthostatictremor.org/).
There are physical aids and certain lifestyle changes that may be helpful in patients with mild OT. Physical aids may offer some symptomatic relief. For instance, portable stools may permit patients to sit rather than have to stand when they are waiting in line or are at social events. A tripod walking stick could be also helpful for this purpose. Furthermore, weight reduction may be helpful in overweight patients.
Pharmacological agents
As a result of the rarity of this condition, there are no well-designed randomized controlled trials. Further, overall, pharmacological agents, which have been empirically used, have often yielded insufficient benefit. Table 3 shows the dosage, clinical efficacy, and practical comments about the different pharmacological agents that have been used to treat OT. Such treatments have side effects, and it is important to carefully consider in each patient whether the benefits outweigh any side effects. As a general rule, treatment should be initiated when the tremor begins to interfere with the patient’s ability to perform daily activities. Surgery may be the final option for a select group of patients who have not responded adequately to medications.7 Unlike essential tremor, OT does not improve either with alcohol or propranolol.40,56
Table 3. Pharmacological Agents to Treat Orthostatic Tremor.
| Medication | Dosage | Clinical Efficacy | Comment |
|---|---|---|---|
| Clonazepam | 0.5–6 mg/day | +++ | Documented effect |
| Gabapentin | 300–2400 mg/day | ++ | Documented effect |
| Levodopa | 300–800 mg/day | ++ | Only short-term benefit |
| Pramipexole | 0.75 mg/day | + | Anecdotal effect |
| Primidone | 125–250 mg/day | + | Anecdotal effect |
| Valproic acid | 500–1000 mg/day | +/– | Anecdotal effect |
| Carbamazepine | 400 mg/day | +/– | Anecdotal effect |
| Phenobarbital | 100 mg/day | +/– | Anecdotal effect |
| Intravenous immunoglobulin1 | 2 g/kg over 3 days | + | Anecdotal effect |
| Propanolol | 120 mg/day | – | Without effect |
| Levetiracetam | 3000 mg/day | – | Without effect |
| Botulinum toxin | 200 mU in the tibialis anterior bilaterally | – | Without effect |
| Alcohol | – | – | Without effect |
It was used in a case of slow orthostatic tremor associated with a novel antineuronal antibody.103
Of all the medications, clonazepam is probably the first-line medication in the treatment of primary and secondary OT.5,10,23,39 This drug reduces tremors in about one-third of people who have the disorder. In some patients, it eliminates tremor almost entirely.95 Clonazepam is typically started at 0.5 mg daily, preferably at night, and, if tolerated, gradually titrated upwards to 2 mg three times a day. However, it is not clear whether this benefit is sustained over time.5,13,55,63,96,97
Second-line therapies, either as monotherapy or in combination, include gabapentin in doses ranging from 300 to 2,400 mg/day,73,98–100 and others with variable benefit, such as primidone,85,96,101 sodium valproate,13 carbamazepine,5 phenobarbital,102 and intravenous immunoglobulin.103 Dopaminergic drugs may be helpful in some patients over a short period of time, especially those who subsequently develop Parkinson’s disease.40 Pramipexole, a dopaminergic agonist, was reported to be effective in a single patient with OT.86 In a series of eight OT patients treated with levodopa, five of them experienced benefit and elected to remain on long-term treatment.43 By contrast, a 2-month open-label trial of levodopa treatment (600 mg/day) led to a small improvement in two of five patients but no significant overall change and no sustained benefit.87
Finally, other medications, such as levetiracetam, are ineffective in the treatment of OT.104 Treatment with 200 mU of botulinum toxin in the tibialis anterior bilaterally did not alleviate either subjective experience of unsteadiness or tremor in a small series of seven OT patients.105
Surgical treatment
The number of OT patients who have undergone surgery to treat their symptoms is still insufficient to make strong recommendations. However, bilateral ventral intermediate thalamic nucleus stimulation could provide long-lasting relief of symptoms in some patients with medication-resistant OT.10,78–84 Nonetheless, in another patient who was treated with unilateral deep brain stimulation of the ventral intermediate thalamic nucleus, clinical benefits receded after 3 months.78 Alternatively, chronic spinal cord stimulation has demonstrated a beneficial effect with long-term follow-up in a few patients with medically intractable primary OT.106,107 Espay et al.,78 postulated that since thalamic and spinal stimulation produce similar results in OT, perhaps a common modulatory role of the cerebello-thalamo-cortico-spinal system may be affected at different levels of the neural axis.
Conclusion
OT is a rare and singular movement disorder that is characterized by tremor of the legs and trunk, and is present on standing and improving on walking or sitting. The origin and mechanism of this condition are not well understood; notwithstanding, most studies suggest that it arises from an oscillator in the posterior fossa, possibly in the brainstem and/or the cerebellum. Although OT is generally considered to be a distinct and primarily “idiopathic” disorder, with normal brain MRI and laboratory work-up, symptomatic OT cases have been described as well.
We are now seeing the clinical expansion of the concept of OT in recent years to include other neurological features (cerebellar dysfunction signs) and non-motor features (cognitive problems, psychiatric problems), the heterogeneity of pharmacological response profiles and clinical progression, and the association of OT with Parkinson’s disease and other types of parkinsonism. We propose that OT might be a family of diseases, unified by the presence of leg tremor, but further characterized by etiological and clinical heterogeneity. Effective pharmacological treatments for the disorder remain limited, although new insights into disease mechanisms might result in more-effective therapies. Along with this, advanced neuroimaging techniques are now demonstrating distinct anatomical and functional changes, some of which are consistent with neuronal loss, in OT patients. However, more work is needed to understand the pathogenesis of this condition.
Footnotes
Funding: Dr. Benito-León is supported by the National Institutes of Health, Bethesda, MD, USA (NINDS #R01 NS39422), the Commission of the European Union (grant ICT-2011-287739, NeuroTREMOR), and the Spanish Health Research Agency (grant FIS PI12/01602).
Financial Disclosures: None.
Conflict of Interest: The authors report no conflict of interest.
Ethics Statement: This study was reviewed by the authors’ institutional ethics committee and was considered exempted from further review.
References
- 1.Benito-León J, Porta-Etessam J. Shaky-leg syndrome and vitamin B12 deficiency. N Engl J Med. 2000;342:981. doi: 10.1056/NEJM200003303421318. doi: 10.1056/NEJM200003303421318. [DOI] [PubMed] [Google Scholar]
- 2.Heilman KM. Orthostatic tremor. Arch Neurol. 1984;41:880–881. doi: 10.1001/archneur.1984.04050190086020. doi: 10.1001/archneur.1984.04050190086020. [DOI] [PubMed] [Google Scholar]
- 3.Pazzaglia P, Sabattini L, Lugaresi E. [On an unusual disorder of erect standing position (observation of 3 cases)] Riv Sper Freniatr Med Leg Alien Ment. 1970;94:450–457. [PubMed] [Google Scholar]
- 4.Deuschl G, Bain P, Brin M. Consensus statement of the Movement Disorder Society on Tremor. Ad Hoc Scientific Committee. Mov Disord. 1998;13(Suppl. 3):2–23. doi: 10.1002/mds.870131303. doi: 10.1002/mds.870131303. [DOI] [PubMed] [Google Scholar]
- 5.Gerschlager W, Munchau A, Katzenschlager R, et al. Natural history and syndromic associations of orthostatic tremor: a review of 41 patients. Mov Disord. 2004;19:788–795. doi: 10.1002/mds.20132. doi: 10.1002/mds.20132. [DOI] [PubMed] [Google Scholar]
- 6.Mestre TA, Lang AE, Ferreira JJ, et al. Associated movement disorders in orthostatic tremor. J Neurol Neurosurg Psychiatry. 2012;83:725–729. doi: 10.1136/jnnp-2012-302436. doi: 10.1001/archneur.1984.04050190086020. [DOI] [PubMed] [Google Scholar]
- 7.Labiano-Fontcuberta A, Benito-León J, Domínguez-González C. [Orthostatic tremor: an enigmatic condition] Rev Neurol. 2012;54:425–434. [PubMed] [Google Scholar]
- 8.Benito-León J, Bermejo-Pareja F, Louis ED, Neurological Disorders in Central Spain Study Group Incidence of essential tremor in three elderly populations of central Spain. Neurology. 2005;64:1721–1725. doi: 10.1212/01.WNL.0000161852.70374.01. doi: 10.1212/01.WNL.0000161852.70374.01. [DOI] [PubMed] [Google Scholar]
- 9.Ganos C, Maugest L, Apartis E, et al. The long-term outcome of orthostatic tremor. J Neurol Neurosurg Psychiatry. 2016;87:167–167. doi: 10.1136/jnnp-2014-309942. doi: 10.1001/archneur.1984.04050190086020. [DOI] [PubMed] [Google Scholar]
- 10.Hassan A, Ahlskog JE, Matsumoto JY, Milber JM, Bower JH, Wilkinson JR. Orthostatic tremor: clinical, electrophysiologic, and treatment findings in 184 patients. Neurology. 2016;86:458–464. doi: 10.1212/WNL.0000000000002328. doi: 10.1212/WNL.0000000000002328. [DOI] [PubMed] [Google Scholar]
- 11.Rigby HB, Rigby MH, Caviness JN. Orthostatic tremor: a spectrum of fast and slow frequencies or distinct entities. Tremor Other Hyperkinet Mov. 2015;5 doi: 10.7916/D8S75FHK. doi: 10.7916/D8S75FHK. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Benito-León J, Louis ED, Puertas-Martín V, et al. Cognitive and neuropsychiatric features of orthostatic tremor: A case-control comparison. J Neurolog Sci. 2016;361:137–143. doi: 10.1016/j.jns.2015.12.031. doi: 10.1016/j.jns.2015.12.031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Piboolnurak P, Yu QP, Pullman SL. Clinical and neurophysiologic spectrum of orthostatic tremor: case series of 26 subjects. Mov Disord. 2005;20:1455–1461. doi: 10.1002/mds.20588. doi: 10.1002/mds.20588. [DOI] [PubMed] [Google Scholar]
- 14.Contarino MF, Welter ML, Agid Y, Hartmann A. Orthostatic tremor in monozygotic twins. Neurology. 2006;66:1600–1601. doi: 10.1212/01.wnl.0000216263.23642.db. doi: 10.1212/01.wnl.0000216263.23642.db. [DOI] [PubMed] [Google Scholar]
- 15.Fischer M, Kress W, Reiners K, Rieckmann P. Orthostatic tremor in three brothers. J Neurol. 2007;254:1759–1760. doi: 10.1007/s00415-007-0647-z. doi: 10.1007/s00415-007-0647-z. [DOI] [PubMed] [Google Scholar]
- 16.Virmani T, Louis ED, Waters C, Pullman SL. Familial orthostatic tremor: an additional report in siblings. Neurology. 2012;79:288–289. doi: 10.1212/WNL.0b013e31825fdfb3. doi: 10.1212/WNL.0b013e31825fdfb3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Bhattacharyya KB, Das D. Familial orthostatic tremor and essential tremor in two young brothers: a rare entity. Ann Indian Acad Neurol. 2013;16:276–278. doi: 10.4103/0972-2327.112497. doi: 10.4103/0972-2327.112497. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Yaltho TC, Ondo WG. Orthostatic tremor: a review of 45 cases. Parkinsonism Relat Disord. 2014;20:723–725. doi: 10.1016/j.parkreldis.2014.03.013. doi: 10.1016/j.parkreldis.2014.03.013. [DOI] [PubMed] [Google Scholar]
- 19.Milone M, Klassen BT, Landsverk ML, Haas RH, Wong LJ. Orthostatic tremor, progressive external ophthalmoplegia, and Twinkle. JAMA Neurol. 2013;70:1429–1431. doi: 10.1001/jamaneurol.2013.3521. doi: 10.1001/jamaneurol.2013.3521. [DOI] [PubMed] [Google Scholar]
- 20.Erro R, Cordivari C, Bhatia KP. SPG31 presenting with orthostatic tremor. Eur J Neurol. 2014;21:e34–35. doi: 10.1111/ene.12360. doi: 10.1111/ene.12360. [DOI] [PubMed] [Google Scholar]
- 21.Gabellini AS, Martinelli P, Gulli MR, Ambrosetto G, Ciucci G, Lugaresi E. Orthostatic tremor: essential and symptomatic cases. Acta Neurolog Scand. 1990;81:113–117. doi: 10.1111/j.1600-0404.1990.tb00944.x. doi: 10.1111/j.1600-0404.1990.tb00944.x. [DOI] [PubMed] [Google Scholar]
- 22.Sanitate SS, Meerschaert JR. Orthostatic tremor: delayed onset following head trauma. Arch Phys Med Rehabil. 1993;74:886–889. doi: 10.1016/0003-9993(93)90017-5. doi: 10.1016/0003-9993(93)90017-5. [DOI] [PubMed] [Google Scholar]
- 23.Benito-León J, Rodríguez J, Ortí-Pareja M, Ayuso-Peralta L, Jiménez-Jiménez FJ, Molina JA. Symptomatic orthostatic tremor in pontine lesions. Neurology. 1997;49:1439–1441. doi: 10.1212/wnl.49.5.1439. doi: 10.1212/WNL.49.5.1439. [DOI] [PubMed] [Google Scholar]
- 24.Vetrugno R, D’Angelo R, Alessandria M, Mascalchi M, Montagna P. Orthostatic tremor in a left midbrain lesion. Mov Disord. 2010;25:793–795. doi: 10.1002/mds.23018. doi: 10.1002/mds.23018. [DOI] [PubMed] [Google Scholar]
- 25.Setta F, Jacquy J, Hildebrand J, Manto MU. Orthostatic tremor associated with cerebellar ataxia. J Neurol. 1998;245:299–302. doi: 10.1007/s004150050222. doi: 10.1007/s004150050222. [DOI] [PubMed] [Google Scholar]
- 26.Manto MU, Setta F, Legros B, Jacquy J, Godaux E. Resetting of orthostatic tremor associated with cerebellar cortical atrophy by transcranial magnetic stimulation. Arch Neurol. 1999;56:1497–1500. doi: 10.1001/archneur.56.12.1497. doi: 10.1001/archneur.56.12.1497. [DOI] [PubMed] [Google Scholar]
- 27.Sarva H, Severt WL, Jacoby N, Pullman SL, Saunders-Pullman R. Secondary orthostatic tremor in the setting of cerebellar degeneration. J Clin Neurosci. 2016;27:173–178. doi: 10.1016/j.jocn.2015.10.027. doi: 10.1016/j.jocn.2015.10.027. [DOI] [PubMed] [Google Scholar]
- 28.Lee HM, Kwon DY, Park MH, Koh SB, Kim SH. Symptomatic orthostatic tremor with progressive cognitive impairment in spinal cord lesions. Clin neurol Neurosurg. 2012;114:1329–1331. doi: 10.1016/j.clineuro.2012.03.021. doi: 10.1016/j.clineuro.2012.03.021. [DOI] [PubMed] [Google Scholar]
- 29.Gilhuis HJ, van Ommen HJ, Pannekoek BJ, Sillevis Smitt PA. Paraneoplastic orthostatic tremor associated with small cell lung cancer. Eur Neurol. 2005;54:225–226. doi: 10.1159/000090715. doi: 10.1159/000090715. [DOI] [PubMed] [Google Scholar]
- 30.Vetrugno R, Fabbri M, Antelmi E, D’Angelo R, Rinaldi R. Orthostatic tremor heralding the onset of stiff-person syndrome. Neurology. 2013;81:1361–1362. doi: 10.1212/WNL.0b013e3182a8254f. doi: 10.1212/WNL.0b013e3182a8254f. [DOI] [PubMed] [Google Scholar]
- 31.Baker M, Fisher K, Lai M, Duddy M, Baker S. Slow orthostatic tremor in multiple sclerosis. Mov Disord. 2009;24:1550–1553. doi: 10.1002/mds.22630. doi: 10.1002/mds.22630. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Tan EK, Lo YL, Chan LL. Graves disease and isolated orthostatic tremor. Neurology. 2008;70((Pt 2)):1497–1498. doi: 10.1212/01.wnl.0000310405.36026.92. doi: 10.1212/01.wnl.0000310405.36026.92. [DOI] [PubMed] [Google Scholar]
- 33.Lin FC, Wu MN, Chen CH, Huang P. Slow orthostatic tremor as the first manifestation of Grave’s disease. Mov Disord. 2013;28:1158–1159. doi: 10.1002/mds.25313. doi: 10.1002/mds.25313. [DOI] [PubMed] [Google Scholar]
- 34.Mazzucchi S, Frosini D, Calabrese R, Bonuccelli U, Ceravolo R. Symptomatic orthostatic tremor associated with Graves’ disease. Neurolog Sci. 2014;35:929–931. doi: 10.1007/s10072-014-1672-1. doi: 10.1007/s10072-014-1672-1. [DOI] [PubMed] [Google Scholar]
- 35.Stich O, Fritzsch C, Heimbach B, Rijntjes M. Orthostatic tremor associated with biclonal IgG and IgA lambda gammopathy of undetermined significance. Mov Disord. 2009;24:154–155. doi: 10.1002/mds.22200. doi: 10.1002/mds.22200. [DOI] [PubMed] [Google Scholar]
- 36.Nasrallah KM, Mitsias PD. Orthostatic tremor due to thiamine deficiency. Mov Disord. 2007;22:440–441. doi: 10.1002/mds.21193. doi: 10.1002/mds.21193. [DOI] [PubMed] [Google Scholar]
- 37.Cruz Tabuenca H, Camacho Velasquez JL, Rivero Sanz E, Sánchez Valiente S, López Del Val J. Orthostatic tremor secondary to recreational use of solvents. Neurologia. 2015 doi: 10.1016/j.nrl.2015.10.007. Dec 14. doi: 10.1016/j.nrl.2015.10.007. [DOI] [PubMed] [Google Scholar]
- 38.Alonso-Navarro H, Ortí-Pareja M, Jiménez-Jiménez FJ, Zurdo-Hernández JM, de Toledo M, Puertas-Muñoz I. [Orthostatic tremor induced by pharmaceuticals] Rev Neurol. 2004;39:834–836. [PubMed] [Google Scholar]
- 39.Jones L, Bain PG. Orthostatic tremor. Pract Neurol. 2011;11:240–243. doi: 10.1136/practneurol-2011-000022. doi: 10.1136/practneurol-2011-000022. [DOI] [PubMed] [Google Scholar]
- 40.Gerschlager W, Brown P. Orthostatic tremor—a review. Hand Clin Neurol. 2011;100:457–462. doi: 10.1016/B978-0-444-52014-2.00035-5. doi: 10.1016/B978-0-444-52014-2.00035-5. [DOI] [PubMed] [Google Scholar]
- 41.Feil K, Bottcher N, Guri F, et al. Long-term course of orthostatic tremor in serial posturographic measurement. Parkinsonism Relat Disord. 2015;21:905–910. doi: 10.1016/j.parkreldis.2015.05.021. doi: 10.1016/j.parkreldis.2015.05.021. [DOI] [PubMed] [Google Scholar]
- 42.Koster B, Lauk M, Timmer J, et al. Involvement of cranial muscles and high intermuscular coherence in orthostatic tremor. Ann Neurol. 1999;45:384–388. doi: 10.1002/1531-8249(199903)45:3<384::aid-ana15>3.0.co;2-j. doi: 10.1002/1531-8249(199903)45:3<384:AID-ANA15>3.0.CO;2-J. [DOI] [PubMed] [Google Scholar]
- 43.Wills AJ, Brusa L, Wang HC, Brown P, Marsden CD. Levodopa may improve orthostatic tremor: case report and trial of treatment. J Neurol, Neurosurg Psychiatry. 1999;66:681–684. doi: 10.1136/jnnp.66.5.681. doi: 10.1136/jnnp.66.5.681. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.de Bie RM, Chen R, Lang AE. Orthostatic tremor in progressive supranuclear palsy. Mov Disord. 2007;22:1192–1194. doi: 10.1002/mds.21434. doi: 10.1002/mds.21434. [DOI] [PubMed] [Google Scholar]
- 45.Apartis E, Tison F, Arne P, Jedynak CP, Vidailhet M. Fast orthostatic tremor in Parkinson's disease mimicking primary orthostatic tremor. Mov Disord. 2001;16:1133–1136. doi: 10.1002/mds.1218. doi: 10.1002/mds.1218. [DOI] [PubMed] [Google Scholar]
- 46.Leu-Semenescu S, Roze E, Vidailhet M, et al. Myoclonus or tremor in orthostatism: an under-recognized cause of unsteadiness in Parkinson's disease. Mov Disord. 2007;22:2063–2069. doi: 10.1002/mds.21651. doi: 10.1002/mds.21651. [DOI] [PubMed] [Google Scholar]
- 47.Ramtahal J, Larner AJ. Shaky legs? Age Ageing. 2009;38:352–353. doi: 10.1093/ageing/afp008. doi: 10.1093/ageing/afp008. [DOI] [PubMed] [Google Scholar]
- 48.Brown P. New clinical sign for orthostatic tremor. Lancet. 1995;346:306–307. doi: 10.1016/s0140-6736(95)92190-7. doi: 10.1016/S0140-6736(95)92190-7. [DOI] [PubMed] [Google Scholar]
- 49.Littmann L. Fact or artifact? The electrocardiographic diagnosis of orthostatic tremor. J Electrocardiol. 2010;43:270–273. doi: 10.1016/j.jelectrocard.2009.11.006. doi: 10.1016/j.jelectrocard.2009.11.006. [DOI] [PubMed] [Google Scholar]
- 50.Pfeiffer G, Hinse P, Humbert T, Riemer G. Neurophysiology of orthostatic tremor. Influence of transcranial magnetic stimulation. Electromyogr Clin Neurophysiol. 1999;39:49–53. [PubMed] [Google Scholar]
- 51.Sharott A, Marsden J, Brown P. Primary orthostatic tremor is an exaggeration of a physiological response to instability. Mov Disord. 2003;18:195–199. doi: 10.1002/mds.10324. doi: 10.1002/mds.10324. [DOI] [PubMed] [Google Scholar]
- 52.Benito-León J, Louis ED. Essential tremor: emerging views of a common disorder. Nat Clin Pract Neurol. 2006;2:666–678. doi: 10.1038/ncpneuro0347. quiz 2p following 91. doi: 10.1038/ncpneuro0347. [DOI] [PubMed] [Google Scholar]
- 53.Benito-León J, Louis ED. Update on essential tremor. Minerva Medica. 2011;102:417–439. [PubMed] [Google Scholar]
- 54.McAuley JH, Britton TC, Rothwell JC, Findley LJ, Marsden CD. The timing of primary orthostatic tremor bursts has a task-specific plasticity. Brain. 2000;123((Pt 2)):254–266. doi: 10.1093/brain/123.2.254. doi: 10.1093/brain/123.2.254. [DOI] [PubMed] [Google Scholar]
- 55.Britton TC, Thompson PD, van der Kamp W, et al. Primary orthostatic tremor: further observations in six cases. J Neurol. 1992;239:209–217. doi: 10.1007/BF00839142. doi: 10.1007/BF00839142. [DOI] [PubMed] [Google Scholar]
- 56.Willeit J, Deisenhammer F, Ransmayr G, Gerstenbrand F. [Orthostatic tremor] Dtsch Med Wochenschr. 1991;116:1509–1512. doi: 10.1055/s-2008-1063781. doi: 10.1055/s-2008-1063781. [DOI] [PubMed] [Google Scholar]
- 57.Glass GA, Ahlskog JE, Matsumoto JY. Orthostatic myoclonus: a contributor to gait decline in selected elderly. Neurology. 2007;68:1826–1830. doi: 10.1212/01.wnl.0000260225.46732.af. doi: 10.1212/01.wnl.0000260225.46732.af. [DOI] [PubMed] [Google Scholar]
- 58.Gasca-Salas C, Arcocha J, Artieda J, Pastor P. Orthostatic myoclonus: an underrecognized cause of unsteadiness. Parkinsonism Relat Disord. 2013;19:1013–1017. doi: 10.1016/j.parkreldis.2013.07.004. doi: 10.1016/j.parkreldis.2013.07.004. [DOI] [PubMed] [Google Scholar]
- 59.Kim JS, Lee MC. Leg tremor mimicking orthostatic tremor as an initial manifestation of Parkinson’s disease. Mov Disord. 1993;8:397–398. doi: 10.1002/mds.870080331. doi: 10.1002/mds.870080331. [DOI] [PubMed] [Google Scholar]
- 60.Thomas A, Bonanni L, Antonini A, Barone P, Onofrj M. Dopa-responsive pseudo-orthostatic tremor in parkinsonism. Mov Disord. 2007;22:1652–1656. doi: 10.1002/mds.21621. doi: 10.1002/mds.21621. [DOI] [PubMed] [Google Scholar]
- 61.Wee AS, Subramony SH, Currier RD. “Orthostatic tremor” in familial-essential tremor. Neurology. 1986;36:1241–1245. doi: 10.1212/wnl.36.9.1241. doi: 10.1212/WNL.36.9.1241. [DOI] [PubMed] [Google Scholar]
- 62.Williams ER, Jones RE, Baker SN, Baker MR. Slow orthostatic tremor can persist when walking backward. Mov Disord. 2010;25:795–797. doi: 10.1002/mds.23024. doi: 10.1002/mds.23024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Uncini A, Onofrj M, Basciani M, Cutarella R, Gambi D. Orthostatic tremor: report of two cases and an electrophysiological study. Acta Neurolog Scand. 1989;79:119–122. doi: 10.1111/j.1600-0404.1989.tb03722.x. doi: 10.1111/j.1600-0404.1989.tb03722.x. [DOI] [PubMed] [Google Scholar]
- 64.Coffeng SM, Hoff JI, Tromp SC. A slow orthostatic tremor of primary origin. Tremor Other Hyperkinet Mov. 2013;3 doi: 10.7916/D8057DNW. doi: 10.7916/D8057DNW. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Kim JS, Lee MC. Leg tremor mimicking orthostatic tremor as an initial manifestation of Parkinson’s disease. Mov Disord. 1993;8:397–398. doi: 10.1002/mds.870080331. doi: 10.1002/mds.870080331. [DOI] [PubMed] [Google Scholar]
- 66.Gallea C, Popa T, Garcia-Lorenzo D, et al. Orthostatic tremor: a cerebellar pathology? Brain. 2016;139((Pt 8)):2182–2197. doi: 10.1093/brain/aww140. doi: 10.1093/brain/aww140. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Schmahmann JD. From movement to thought: anatomic substrates of the cerebellar contribution to cognitive processing. Hum Brain Mapp. 1996;4:174–198. doi: 10.1002/(SICI)1097-0193(1996)4:3<174::AID-HBM3>3.0.CO;2-0. doi: 10.1002/(SICI)1097-0193(1996)4:3<174::AID-HBM3>3.0.CO;2-0. [DOI] [PubMed] [Google Scholar]
- 68.Benito-León J, Louis ED, Manzanedo E, et al. Resting state functional MRI reveals abnormal network connectivity in orthostatic tremor. Medicine. 2016;95:e4310. doi: 10.1097/MD.0000000000004310. doi: 10.1097/MD.0000000000004310. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Benito-León J, Morales JM, Rivera-Navarro J. Health-related quality of life and its relationship to cognitive and emotional functioning in multiple sclerosis patients. Eur J Neurol. 2002;9:497–502. doi: 10.1046/j.1468-1331.2002.00450.x. doi: 10.1046/j.1468-1331.2002.00450.x. [DOI] [PubMed] [Google Scholar]
- 70.Martínez-Martín P, Benito-León J, Alonso F, Catalán MJ, Pondal M, Zamarbide I. Health-related quality of life evaluation by proxy in Parkinson's disease: approach using PDQ-8 and EuroQoL-5D. Mov Disord. 2004;19:312–318. doi: 10.1002/mds.10656. doi: 10.1002/mds.10656. [DOI] [PubMed] [Google Scholar]
- 71.Martínez-Martín P, Jiménez-Jiménez FJ, Carroza García E, et al. Most of the Quality of Life in Essential Tremor Questionnaire (QUEST) psychometric properties resulted in satisfactory values. J Clin Epidemiol. 2010;63:767–773. doi: 10.1016/j.jclinepi.2009.09.001. doi: 10.1016/j.jclinepi.2009.09.001. [DOI] [PubMed] [Google Scholar]
- 72.Gerschlager W, Katzenschlager R, Schrag A, et al. Quality of life in patients with orthostatic tremor. J Neurol. 2003;250:212–215. doi: 10.1007/s00415-003-0980-9. doi: 10.1007/s00415-003-0980-9. [DOI] [PubMed] [Google Scholar]
- 73.Rodrigues JP, Edwards DJ, Walters SE, Byrnes ML, Thickbroom G, Stell R, et al. Gabapentin can improve postural stability and quality of life in primary orthostatic tremor. Mov Disord. 2005;20:865–870. doi: 10.1002/mds.20392. doi: 10.1002/mds.20392. [DOI] [PubMed] [Google Scholar]
- 74.Wu YR, Ashby P, Lang AE. Orthostatic tremor arises from an oscillator in the posterior fossa. Mov Disord. 2001;16:272–279. doi: 10.1002/mds.1045. doi: 10.1002/mds.1045. [DOI] [PubMed] [Google Scholar]
- 75.Spiegel J, Fuss G, Krick C, Dillmann U. Impact of different stimulation types on orthostatic tremor. Clin Neurophysiol. 2004;115:569–575. doi: 10.1016/j.clinph.2003.10.013. doi: 10.1016/j.clinph.2003.10.013. [DOI] [PubMed] [Google Scholar]
- 76.Muthuraman M, Hellriegel H, Paschen S, et al. The central oscillatory network of orthostatic tremor. Mov Disord. 2013;28:1424–1430. doi: 10.1002/mds.25616. doi: 10.1002/mds.25616. [DOI] [PubMed] [Google Scholar]
- 77.Wills AJ, Thompson PD, Findley LJ, Brooks DJ. A positron emission tomography study of primary orthostatic tremor. Neurology. 1996;46:747–752. doi: 10.1212/wnl.46.3.747. doi: 10.1212/WNL.46.3.747. [DOI] [PubMed] [Google Scholar]
- 78.Espay AJ, Duker AP, Chen R, et al. Deep brain stimulation of the ventral intermediate nucleus of the thalamus in medically refractory orthostatic tremor: preliminary observations. Mov Disord. 2008;23:2357–2362. doi: 10.1002/mds.22271. doi: 10.1002/mds.22271. [DOI] [PubMed] [Google Scholar]
- 79.Guridi J, Rodríguez-Oroz MC, Arbizu J, et al. Successful thalamic deep brain stimulation for orthostatic tremor. Mov Disord. 2008;23:1808–1811. doi: 10.1002/mds.22001. doi: 10.1002/mds.22001. [DOI] [PubMed] [Google Scholar]
- 80.Magariños-Ascone C, Ruiz FM, Millan AS, et al. Electrophysiological evaluation of thalamic DBS for orthostatic tremor. Mov Disord. 2010;25:2476–2477. doi: 10.1002/mds.23333. doi: 10.1002/mds.23333. [DOI] [PubMed] [Google Scholar]
- 81.Yaltho TC, Ondo WG. Thalamic deep brain stimulation for orthostatic tremor. Tremor Other Hyperkinet Mov. 2011;1 doi: 10.7916/D8NZ86C1. doi: 10.7916/D8NZ86C1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Lyons MK, Behbahani M, Boucher OK, Caviness JN, Evidente VG. Orthostatic tremor responds to bilateral thalamic deep brain stimulation. Tremor Other Hyperkinet Mov. 2012;2 doi: 10.7916/D8TQ608K. doi: 10.7916/D8TQ608K. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Contarino MF, Bour LJ, Schuurman PR, et al. Thalamic deep brain stimulation for orthostatic tremor: Clinical and neurophysiological correlates. Parkinsonism Relat Disord. 2015;21:1005–1007. doi: 10.1016/j.parkreldis.2015.06.008. doi: 10.1016/j.parkreldis.2015.06.008. [DOI] [PubMed] [Google Scholar]
- 84.Coleman RR, Starr PA, Katz M, et al. Bilateral ventral intermediate nucleus thalamic deep brain stimulation in orthostatic tremor. Stereotact Funct Neurosurg. 2016;94:69–74. doi: 10.1159/000444127. doi: 10.1159/000444127. [DOI] [PubMed] [Google Scholar]
- 85.FitzGerald PM, Jankovic J. Orthostatic tremor: an association with essential tremor. Mov Disord. 1991;6:60–64. doi: 10.1002/mds.870060111. doi: 10.1002/mds.870060111. [DOI] [PubMed] [Google Scholar]
- 86.Finkel MF. Pramipexole is a possible effective treatment for primary orthostatic tremor (shaky leg syndrome) Arch Neurol. 2000;57:1519–1520. doi: 10.1001/archneur.57.10.1519. doi: 10.1001/archneur.57.10.1519. [DOI] [PubMed] [Google Scholar]
- 87.Katzenschlager R, Costa D, Gerschlager W, et al. [123I]-FP-CIT-SPECT demonstrates dopaminergic deficit in orthostatic tremor. Ann Neurol. 2003;53:489–496. doi: 10.1002/ana.10475. doi: 10.1002/ana.10475. [DOI] [PubMed] [Google Scholar]
- 88.Spiegel J, Behnke S, Fuss G, Becker G, Dillmann U. Echogenic substantia nigra in patients with orthostatic tremor. J Neural Trans. 2005;112:915–920. doi: 10.1007/s00702-004-0236-6. doi: 10.1007/s00702-004-0236-6. [DOI] [PubMed] [Google Scholar]
- 89.Vaamonde J, García A, Flores JM, Ibáñez R, Gargallo L. [Study of presynaptic nigrostriatal pathway by 123-I-FD-CIT-SPECT (DatSCAN SPECT) in primary orthostatic tremor] Neurologia. 2006;21:37–39. [PubMed] [Google Scholar]
- 90.Trocello JM, Zanotti-Fregonara P, Roze E, et al. Dopaminergic deficit is not the rule in orthostatic tremor. Mov Disord. 2008;23:1733–1738. doi: 10.1002/mds.22224. doi: 10.1002/mds.22224. [DOI] [PubMed] [Google Scholar]
- 91.Wegner F, Strecker K, Boeckler D, et al. Intact serotonergic and dopaminergic systems in two cases of orthostatic tremor. J Neurol. 2008;255:1840–1842. doi: 10.1007/s00415-008-0023-7. doi: 10.1007/s00415-008-0023-7. [DOI] [PubMed] [Google Scholar]
- 92.Benito-León J, Louis ED, Mato-Abad V, et al. In vivo Neurometabolic Profiling in Orthostatic Tremor. Medicine. 2016;95:e4848. doi: 10.1097/MD.0000000000004848. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Benito-León J, Louis ED, Posada IJ, et al. Population-based case-control study of cognitive function in early Parkinson’s disease (NEDICES) J Neurolog Sci. 2011;310:176–182. doi: 10.1016/j.jns.2011.06.054. doi: 10.1016/j.jns.2011.06.054. [DOI] [PubMed] [Google Scholar]
- 94.Benito-León J, Louis ED, Bermejo-Pareja F, Neurological Disorders in Central Spain Study Group Population-based case-control study of cognitive function in essential tremor. Neurology. 2006;66:69–74. doi: 10.1212/01.wnl.0000192393.05850.ec. doi: 10.1212/01.wnl.0000192393.05850.ec. [DOI] [PubMed] [Google Scholar]
- 95.Pradalier A, Apartis E, Vincent D, Campinos C. [Primary orthostatic tremor] Rev Med Intern. 2002;23:193–197. doi: 10.1016/s0248-8663(01)00536-7. doi: 10.1016/S0248-8663(01)00536-7. [DOI] [PubMed] [Google Scholar]
- 96.McManis PG, Sharbrough FW. Orthostatic tremor: clinical and electrophysiologic characteristics. Muscle Nerve. 1993;16:1254–1260. doi: 10.1002/mus.880161117. doi: 10.1002/mus.880161117. [DOI] [PubMed] [Google Scholar]
- 97.Papa SM, Gershanik OS. Orthostatic tremor: an essential tremor variant? Mov Disord. 1988;3:97–108. doi: 10.1002/mds.870030201. doi: 10.1002/mds.870030201. [DOI] [PubMed] [Google Scholar]
- 98.Evidente VG, Adler CH, Caviness JN, Gwinn KA. Effective treatment of orthostatic tremor with gabapentin. Mov Disord. 1998;13:829–831. doi: 10.1002/mds.870130513. doi: 10.1002/mds.870130513. [DOI] [PubMed] [Google Scholar]
- 99.Onofrj M, Thomas A, Paci C, D’Andreamatteo G. Gabapentin in orthostatic tremor: results of a double-blind crossover with placebo in four patients. Neurology. 1998;51:880–882. doi: 10.1212/wnl.51.3.880. doi: 10.1212/WNL.51.3.880. [DOI] [PubMed] [Google Scholar]
- 100.Rodrigues JP, Edwards DJ, Walters SE, et al. Blinded placebo crossover study of gabapentin in primary orthostatic tremor. Mov Disord. 2006;21:900–905. doi: 10.1002/mds.20830. doi: 10.1002/mds.20830. [DOI] [PubMed] [Google Scholar]
- 101.van der Zwan A, Verwey JC, van Gijn J. Relief of orthostatic tremor by primidone. Neurology. 1988;38:1332. doi: 10.1212/wnl.38.8.1332. doi: 10.1212/WNL.38.8.1332. [DOI] [PubMed] [Google Scholar]
- 102.Cabrera-Valdivia F, Jiménez-Jiménez FJ, García Albea E, Tejeiro-Martínez J, Vaquero Ruiperez JA, Ayuso-Peralta L. Orthostatic tremor: successful treatment with phenobarbital. Clin Neuropharmacol. 1991;14:438–441. doi: 10.1097/00002826-199110000-00008. [PubMed] [Google Scholar]
- 103.Hegde M, Glass GA, Dalmau J, Christine CW. A case of slow orthostatic tremor, responsive to intravenous immunoglobulin. Mov Disord. 2011;26:1563–1565. doi: 10.1002/mds.23610. doi: 10.1002/mds.23610. [DOI] [PubMed] [Google Scholar]
- 104.Hellriegel H, Raethjen J, Deuschl G, Volkmann J. Levetiracetam in primary orthostatic tremor: a double-blind placebo-controlled crossover study. Mov Disord. 2011;26:2431–2434. doi: 10.1002/mds.23881. doi: 10.1002/mds.23881. [DOI] [PubMed] [Google Scholar]
- 105.Bertram K, Sirisena D, Cowey M, Hill A, Williams DR. Safety and efficacy of botulinum toxin in primary orthostatic tremor. J Clin Neurosci. 2013;20:1503–1505. doi: 10.1016/j.jocn.2012.12.025. doi: 10.1016/j.jocn.2012.12.025. [DOI] [PubMed] [Google Scholar]
- 106.Krauss JK, Weigel R, Blahak C, et al. Chronic spinal cord stimulation in medically intractable orthostatic tremor. J Neurol Neurosurg Psychiatry. 2006;77:1013–1016. doi: 10.1136/jnnp.2005.086132. doi: 10.1136/jnnp.2005.086132. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107.Blahak C, Sauer T, Baezner H, et al. Long-term follow-up of chronic spinal cord stimulation for medically intractable orthostatic tremor. J Neurol. 2016 doi: 10.1007/s00415-016-8239-4. doi: 10.1007/s00415-016-8239-4. [DOI] [PubMed] [Google Scholar]