Abstract
Retinopathy of prematurity (ROP) is a major and leading cause of blindness in premature infants. It has been realized that early treatment for ROP is important. However, all the early treatments of ROP are focusing on peripheral retinal ablation which does not surmount the limit of extinguishing retinal neovascularization and protecting the retinas of children with ROP from the injury of ablation. In this study, we investigated the morphological changes of retina and oxidative stress alterations in the early phase of oxygen-induced retinopathy (OIR) and tested the effects of 17β-estradiol (17β-E2) , a nonselective estrogen receptor (ER) agonist, on early phase OIR development. We found that large central capillary-free areas were induced in the retinas of pups exposed to hyperoxia on postnatal day 9 (P9) , whereas vascularization was almost complete in the retinas of pups exposed to normoxia at the same age. The concentrations of malondiadehyde (MDA) , an end-product of oxidative stress, and nicotinamide adenine dinucleotide phosphate (NADPH) oxidase, a major enzyme producing free radicals, as well as the activity of NADPH oxidase were significantly elevated in the retinas of pups exposed to hyperoxia on P9 and postnatal day 13 (P13) compared to those in age matched pups exposed to normoxia. Treatment with 17β-E2 decreased not only the percentage of the central capillary-free area to total retina area but also the concentrations of MDA and NADPH oxidase as well as the activity of NADPH oxidase in a dose-dependent manner in pups exposed to hyperoxia on p9 and P13. The concentration of VEGF was significantly decreased on P9 but increased on P14 in the retinas of pups exposed to hyperoxia, whereas it was significantly elevated on P9 but decreased on P14 in the retinas of pups treated with 17β-E2. The effect of 17β-E2 could be reversed by the co-treatment with ICI182780, a high affinity estrogen receptor antagonist, which suggested that 17β-E2 might exert its effect on early hyperoxic phase of OIR through estrogen receptor. Our results suggest that treatment with antioxidant drugs at early hyperoxic phase of ROP even before the appearance of retinal neovascularization may be more effective than their application to ROP at late phase, which may abolish the deleterious factors that contribute to retinal neovascularization and promote retinal blood vessels to develop healthily.
Keywords: oxygen-induced retinopathy, oxidative stress, 17β-estradiol, receptor, NADPH oxidase
INTRODUCTION
Retinopathy of prematurity (ROP) is a disease that occurs in premature infants and is one of the most common causes of visual loss in childhood and can lead to lifetime vision impairment and blindness [1]. It was found that the relatively high levels of oxygen routinely given to premature infants were an important risk factor, and that reducing the level of oxygen given to premature babies reduced the incidence of ROP [2,3]. Although newer technology and methods have been applied to monitor the oxygen levels when rescuing premature infants with oxygen, it is unavoidable for the premature infants to be exposed to relative high levels of oxygen. Therefore, for understanding the mechanisms of ROP, oxygen-induced retinopathy (OIR) is still used to generate ROP in animal models [4].
Although different therapeutic treatments have been discovered, ROP remains as a major cause of blindness in premature infants, and the incidence of ROP is increasing in light of the rising numbers of preterm deliveries and improved neonatal care [1]. At present, most of the therapeutic treatments, including cryotherapy, photocoagulation and powerful anti-neovascular drugs, are focusing on depressing retinal neovascularization [1]. However, residual anatomical changes, the high prevalence of strabismus, amblyopia and high ametropia in those infants, and injury to the vulnerable retina caused by treatments themselves have made it difficult to restore impaired vision function appreciably by inhibiting retinal neovascularization [5–8]. Therefore, optimal treatment for ROP should be prior to retinal neovascularization, indicating that the best time for treating ROP should be in the early hyperoxic phase, not during the hypoxic phase.
OIR in animal models can be divided into an early hyperoxic phase and a late hypoxic phase like ROP [9]. The morphological changes of retina in the early phase of OIR are more obvious than those in the late phase [10], indicating that to explore the mechanism of hyperoxia-induced vascular loss of OIR in the early phase, which is closely related to primary retinal vascular loss and late neovascularization, may help to develop better therapeutic approaches for treating ROP. Oxidative stress plays a pivotal role in OIR. Nicotinamide adenine dinucleotide phosphate (NADPH) oxidase, a major enzyme producing free radicals, is crucial for OIR [11,12]. We and others have found that 17β-estradiol (17β-E2) , a nonselective estrogen receptor (ER) agonist, could effectively ameliorate later- phase OIR and decrease the concentration of malondiadehyde (MDA) , an end-product of oxidative stress, on postnatal day 17 [13,14]. However, the role of 17β-E2 on early hyperoxic phase of OIR has not been investigated. In this study, we examined the morphological changes of retina and oxidative stress alterations in the early hyperoxic phase of OIR and tested the effects of 17β-E2 on OIR development during this phase.
MATERIALS AND METHODS
Oxygen Exposure to Induce Retinopathy in Pups
OIR was induced as described by Smith et al and our previous publication [4,15]. In brief, 7-day-old C57BL/6J pups were randomly assigned to six experimental groups: Group 1, room air with vehicle injection (control) ; Group 2, hyperoxia (75% ± 2% O2) with vehicle injection; Group 3, hyperoxia (75% ± 2% O2) plus 0.1 μg of 17β-E2 (Sigma-Aldrich, St. Louis, MO, USA) injection; Group 4, hyperoxia (75% ± 2% O2) plus 1.0 μg of 17β-E2 injection; Group 5, hyperoxia (75% ± 2% O2) plus 10.0 μg of 17 β-E2 injection; and Group 6, hyperoxia (75% ± 2% O2) plus 10.0 μg of 17β-E2 and 100.0 μg of ICI182780 (an antagonist of estrogen receptors) (Tocris Bioscience, Ellisville, IL, USA) injection. In general, pups were injected subcutaneously with different doses of 17β-E2 (dissolved in ethanol and diluted in 0.05 ml PBS (phosphate-buffered saline) /pup) or vehicle alone from postnatal day 7 (P7) to P13 daily (14) , whereas pups treated with the hyperoxia and 17β-E2 were exposed to hyperoxia (75% ± 2% O2) plus 17β-E2 from P7 to P12 and then exposed to room air from P12 to P13. The pups for normoxia treatment were kept in room air from P7 to P13. The pups were sacrificed on P9 and P13, respectively.
Retinal Flat Mounts and Quantification of the Avascular Area
Retinal flat mounts were obtained by perfusing the pups’ left ventricles with high-molecular-weight dextran (2,000,000 Da) conjugated with fluorescein (Sigma-Aldrich, St. Louis, MO, USA) as previously described [4,15,16]. A fluorescence microscope was applied to capture the images of the superficial blood vessel layers. The total retinal and central capillary-free areas were measured with Optimas software, version 5.2 (Meyer Instruments, Inc., Houston, TX, USA) , and the central capillary-free area was represented as a percentage of the total retinal area.
Measurement of Retinal Lipid Peroxidation Concentrations
The retinal lipid peroxidation concentrations on P9 and P13 were determined with MDA assay kit (Nanjing Jiancheng Bioengineering Ins, Nanjing, China) as described in our previous publications [15, 17,18], and were represented as nanomole of MDA per mg proteins.
Measurement of Retinal NADPH Oxidase Concentrations
The retinal NADPH oxidase concentrations on P9 and P13 were assayed with EnVision immunohistochemistry kit (Genmed Scientifics Inc., Wilmington, DE, USA) as described in our previous publication [15].
Measurement of Retinal NADPH Oxidase Activity
The NADPH oxidase activity in the retinas was determined on P9 and P13 with the NADPH oxidase activity assay kit (Genmed Scientifics Inc., Wilmington, DE, USA) as described in our previous publications [15,19], and was represented as millimoles of NADPH per minute per milligram of proteins.
Measurement of Retinal VEGF Concentrations
VEGF concentrations in the retinas were measured on P9 and P14 with enzyme linked immunosorbent assay (ELISA) kit (Santa Cruz Biotechnology, Inc., Santa Cruz, CA, USA) as described in our previous publication [15].
Statistical analysis
Data were analyzed with GraphPad Prism software, version 5.01 (GraphPad Software Inc., La Jolla, CA, USA) . For each test, a value of P <0.05 was considered statistically significant.
Ethics Statement
All the experiments were approved by the Eye Institutional Committee of Shaanxi Province for Animal Use in Research and Education and conformed to the ARVO Statement for the Use of Animals in Ophthalmic and Vision Research.
RESULTS
Oxidative stress induces retinopathy in the early hyperoxic phase and 17β-E2 treatment blocks oxygen induced OIR at this phase
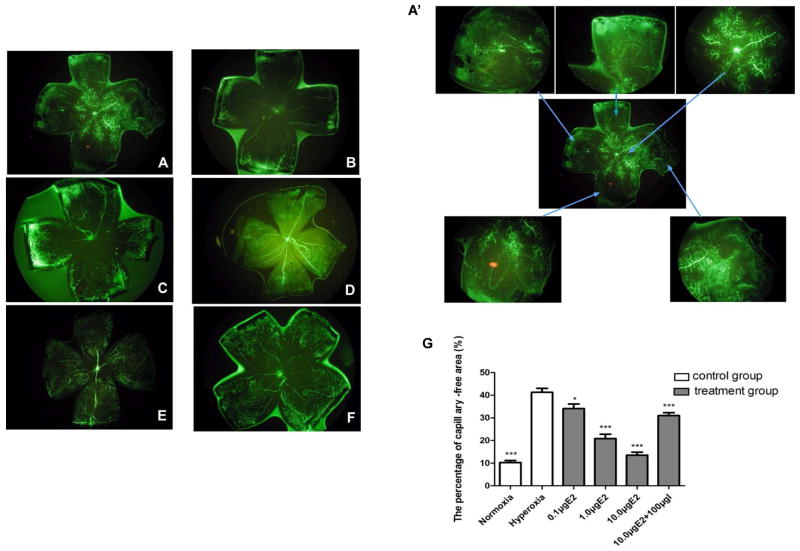
To investigate whether oxidative stress was able to induce retinopathy in the early hyperoxic phase, we exposed pups to normoxia and hyperoxia conditions from postnatal day 7 (P7) to 9 (P9) . We found that pups exposed to hyperoxia induced considerable vascular changes in retina on P9 compared to that in pups exposed to room air. In the normoxia (room air) treated pups (group 1) , retinal vascularization on P9 was almost completed characterized by its maturation in the peripheral zone and partial immaturation in the central zone, (Figs. 1A and 1A′) , whereas large retinal capillary-free areas were found in the central zones of retina in pups exposed to hyperoxia (75% ± 2% O2) condition (group 2) (Fig. 1B) . This result suggested that oxidative stress can induce retinopathy in the early hyperoxic phase. To investigate the effect of 17β-E2 on hyperoxia induced OIR, we treated the pups with hyperoxia plus different concentrations of 17β-E2. We found that in the hyperoxia plus 17β-E2 treated groups (group 3, 4, and 5) , the percentage of the central capillary-free area to total retina area was decreased in a dose-dependent manner, in which treatment with hyperoxia plus 10.0 μg of 17β-E2 had the most striking effect on reducing the central capillary-free area (Fig. 1C, 1D and 1E) (n = 6 per group, p < 0.05 between any two groups) . However, the decrease of the capillary-free area induced by treatment with hyperoxia plus 10.0 μg of 17β-E2 could be blocked in the retinas of the pups co-treated with 100.0 μg of ICI182780, a high affinity estrogen receptor antagonist (group 6) (Fig. 1F) , which suggested that 17β-E2 may be through estrogen receptor to ameliorate oxygen induced OIR at this phase. In addition, no neovascular tufts or clusters were found on the border of the avascular zone and the vascular zone in any of the retina flat mounts. Our statistical analysis indicated that the percentage of the capillary-free area to total retina area between normoxia and hyperoxia groups as well as between hyperoxia alone exposure group and hyperoxia plus different concentrations of 17β-E2 with or without ICI182780 groups were significant changed (n in each group = 6, P < 0.05) (Fig. 1G) .
Fig 1. Retinal flat mounts and the percentage of retinal avascular area on P9.
A. Retinal vascularization was distributed to almost the whole retinas of normoxia control pups. The mature retinal vessels were found in the peripheral region while the immature retinal vessels were found in the central region as showed by the stained vessel wall. B. Large capillary-free areas were found in the retinas of hyperoxia alone exposure pups. C–E. The percentage of capillary-free area was decreased in the retinas of pups treated with hyperoxia plus different concentrations of 17β-E2. F. The capillary-free area was broadened in the retinas of pups treated with hyperoxia plus 10.0 μg of 17β-E2 and 100.0 μg of ICI182780. No neovascular tufts or clusters were found on the border of the avascular region and the vascular region in any of the retinal flat mounts. A′. Magnification of retinal flat mounts of normoxia control pups on P9. The blurred mature vessels in the whole retinal flat mounts of normoxia control pups on P9 obviously appeared under magnification (×4) . G. Statistical analysis of the difference in retinal avascular area represent with percentage of the percentage of the capillary-free area to total retina area between normoxia and hyperoxia groups as well as between hyperoxia alone exposure group and hyperoxia plus different concentrations of 17β-E2 with or without ICI182780 groups (n = 6 in each group) . *P<0.05, *** P<0.01. I is the abbreviation of ICI182780.
Retinal lipid peroxidation characterized by MDA levels was increased in early hyperoxic phase of OIR and could be decreased by 17β-E2 treatment
To evaluate the retinal lipid peroxidation in early hyperoxic phase of OIR, we measured the concentrations of malondiadehyde (MDA) , an end-product of oxidative stress [13,14], in different experimental groups. We first found that MDA levels were significantly increased in the retinas of the hyperoxia-exposed pups compared to those in age-matched normoxia control pups (P < 0.01 on P9 and P < 0.05 on P13) (Fig. 2A and 2B) , whereas MDA levels in the retinas of the normoxia control pups were not significantly changed as analyzed on P9 and on P13 (P > 0.05) . Treatment with different concentrations of 17β-E2 significantly decreased the levels of MDA in the retinas compared to those in age matched pups treated with hyperoxia alone, and the effect of 17β-E2 was more significant on P9 pups than that on P13 pups (F=38.56, P < 0.05 on P9 and F=10.07, P < 0.05 on P13) (Fig. 2A and 2B) . As expectation, the lowest MDA level was found in the retinas of hyperoxia plus 10.0 μg 17β-E2 treated pups (P < 0.01 on P9 and P13) compared to the hyperoxia alone exposed pups although it was still higher than that in the retinas of the normoxia-exposed pups (P < 0.01, on P9 and P13) (Fig. 2A and 2B) . The effect of 17β-E2 on reducing the levels of MDA in hyperoxia plus 17β-E2 treated pups could be markedly reversed by co-treatment with ICI182780 (P < 0.01 on P9 and P < 0.05 on P13, respectively) (Fig. 2A and 2B) .
Fig 2. MDA concentration in the retina on P9 and P13.
A and B. Retinal MDA concentration was significantly increased in hyperoxia alone exposure P9 (A) and P13 (B) pups compared to that in normoxia control pups, and was significantly decreased upon hyperoxia plus different concentrations of 17β-E2 treatment at P9 (A) and P13 (B) pups. No significant difference was found between hyperoxia alone exposure pups and hyperoxia plus 10.0 μg of 17β-E2 and 100.0 μg of ICI182780-treated pups. *P<0.05, ** or *** P<0.01. I is the abbreviation of ICI182780.
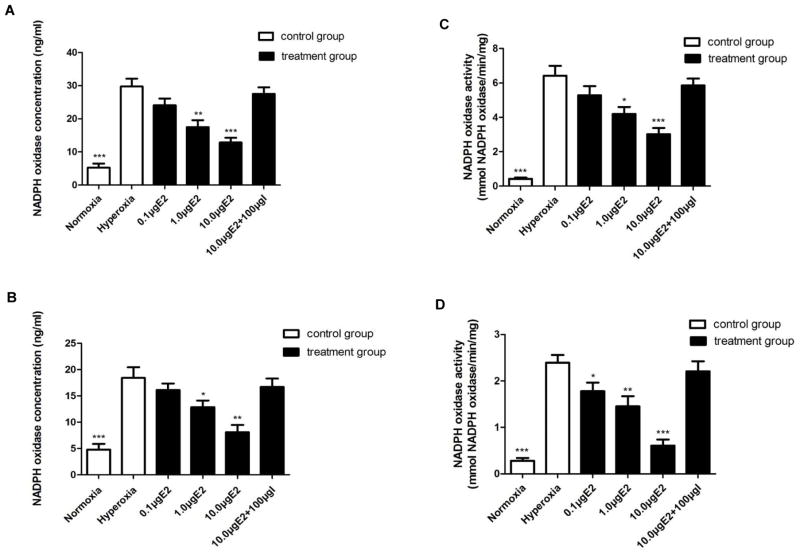
NADPH oxidase concentrations and activity were increased in early OIR and could be normalized by 17β-E2 treatment
It has been reported that NADPH oxidase, a major enzyme producing free radicals, is crucial for OIR [11,12]. We further found that the concentration and activity of NADPH oxidase was not significantly changed in the retinas of the normoxia group on P9 and P13 (P > 0.05) whereas the concentration and activity of NADPH oxidase was increased significantly in the retinas of the hyperoxia alone exposed pups both on P9 and on P13 versus the normoxia control pups (P < 0.01) (Fig. 3) . The concentration and activity of NADPH oxidase were markedly reduced in the retinas of pups treated with hyperoxia plus 17β-E2 compared to that in hyperoxia alone exposed pups. The lowest level of NADPH oxidase was found in the retinas of the pups treated with hyperoxia plus 10.0 μg of 17β-E2, while it could be significantly reversed by co-treatment with ICI182780 on P9 and P13 pups (P < 0.01 both on P9 and on P13) (Fig. 3) .
Fig 3. NADPH oxidase concentration and activity in the retina on P9 and P13.
A and B. Retinal NADPH oxidase concentration was significantly increased in hyperoxia alone exposure P9 (A) and P13 (B) pups compared to that in normoxia control pups, and was significantly decreased upon hyperoxia plus different concentrations of 17β-E2 treatment at P9 (A) and P13 (B) pups. No significant difference was found between hyperoxia alone exposure pups and hyperoxia plus 10.0 μg of 17β-E2 and 100.0 μg of ICI182780-treated pups. *P<0.05, ** or *** P<0.01. I is the abbreviation of ICI182780. C and D. Retinal NADPH oxidase activity was significantly increased in hyperoxia alone exposure P9 (C) and P13 (D) pups compared to that in normoxia control pups, and was significantly decreased upon hyperoxia plus different concentrations of 17β-E2 treatment at P9 (C) and P13 (D) pups. No significant difference was found between hyperoxia alone exposure pups and hyperoxia plus 10.0 μg of 17β-E2 and 100.0 μg of ICI182780-treated pups. *P<0.05, ** or *** P<0.01. I is the abbreviation of ICI182780.
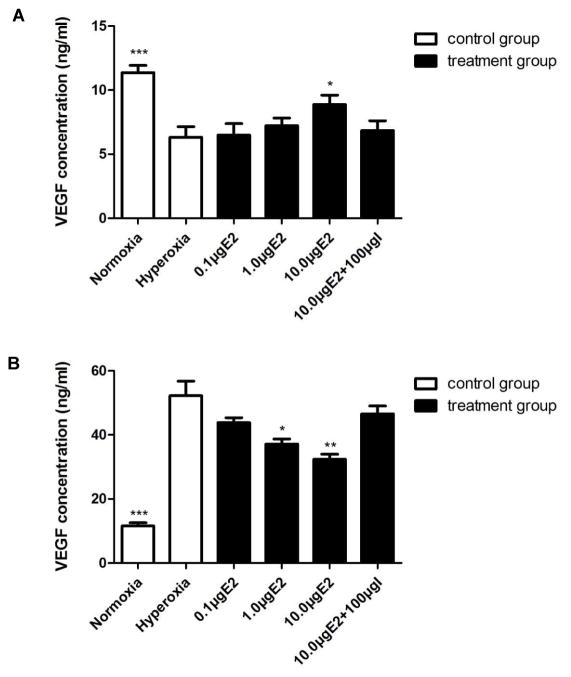
VEGF concentrations were decreased in early stage of early hyperoxic phase of OIR but increased in later stage of early hyperoxic phase of OIR and could be normalized by 17β-E2 treatment at both stages
There was no significant difference of VEGF concentrations was found in the retinas of the normoxia group on P9 and P14 (P > 0.05) , whereas VEGF concentrations were decreased on P9 but increased on P14 in the retinas of the hyperoxia alone exposed pups versus the normoxia control pups (P < 0.01) (Fig. 4A and 4B) . Treatment with different concentrations of 17β-E2 gradually increased VEGF concentrations in retinas in P9 pups compared to that in hyperoxia alone exposed pups, and the highest level was found in 10.0 μg 17β-E2-treated P9 pups (P < 0.05) (Fig. 4A and 4B) . However, increased VEGF concentrations in retinas of pups induced by hyperoxia alone exposure from P7 to P14 could be decreased by the co-treatment with different concentrations of 17β-E2 and the lowest level of VEGF in retinas was found in pups with hyperoxia plus 10.0 μg 17β-E2 treatment versus the hyperoxia alone exposed pups (P < 0.01) (Fig. 4A and 4B) . The VEGF concentrations in the retinas of the 10.0 μg of 17β-E2-treated pups on P9 were not significantly reversed by ICI182780 (P > 0.05, versus the 10.0 μg of 17β-E2-treated group) , while the decreased VEGF concentrations in the retinas of the 10.0 μg of the 17β-E2-treated pups on P14 could be significantly reversed by ICI182780 on P14 (P < 0.01, versus the 10.0 μg of 17β-E2-treated group) (Fig. 4A and 4B) .
Fig 4. VEGF concentration on P9 and P14.
A. Retinal VEGF concentration on P9 was significantly decreased in the hyperoxia alone exposure pups compared to that in the normoxia control pups, and was significantly increased upon hyperoxia plus 10.0 ug of 17β-E2 treatment compared to that in hyperoxia alone exposure pups. *P<0.05, *** P<0.01. I is the abbreviation of ICI182780. B. Retinal VEGF concentration on P14 was significantly increased in the hyperoxia alone exposure pups compared to that in the normoxia control pups, and was markedly decreased in the hyperoxia plus 1.0 and 10.0 ug of 17β-E2 treated pups compared to that in hyperoxia alone exposure pups. No significant difference was found between hyperoxia alone exposure pups and hyperoxia plus 10.0 μg of 17β-E2 and 100.0 μg of ICI182780-treated pups. *P<0.05, ** or *** P<0.01. I is the abbreviation of ICI182780.
DISCUSSION
Due to the complications with the traditional treatment of ROP, researchers have begun to explore the feasibility of early treatment for ROP, and exciting results have been achieved in terms of structural outcomes and graded visual acuity [20]. However, all the early treatments of ROP are focusing on peripheral retinal ablation which does not surmount the limit of extinguishing retinal neovascularization and protecting the retinas of children with ROP from the injury of ablation. We propose that an effective treatment of ROP should be started earlier than the appearance of retinal neovascularization, which may abolish the deleterious factors that contribute to retinal neovascularization and promote retinal blood vessels to develop healthily.
In this study, we found that the retinal vascularization was almost complete on normoxia-exposed pups at P9 while large capillary-free areas were found in the retinas of hyperoxia-exposed pups at the same age, indicating that marked morphological and pathological retinopathy could occur during the first two days of OIR and is consistent with the chronological development of retinal capillary-free areas found by others [10]. The capillary-free areas in the retinas of hyperoxia-exposed pups on P9 were overlapped with the central zones with immature retinal vessels of the retinas of normoxia-exposed pups on P9, suggesting that hyperoxia might only damage immature vessels but not mature vessels during the development of early hyperoxic phase of OIR.
The obvious retinal morphological alterations of OIR in the early hyperoxic phase encouraged us to explore the mechanism of OIR during this phase. We found that MDA and NADPH oxidase concentrations and NADPH activity were increased in the retinas of P9 pups exposed to hyperoxia, which was closely related to oxidative stress and resulted in the formation of capillary-free areas. Although antioxidant drugs were used to treat ROP many years ago [21], however, unsatisfactory clinical results discouraged their further application to ROP [22, 23]. According to our results and other relevant studies [22–24], improper treating time may be responsible for those unsatisfactory results. Thus, our results suggest that treatment with antioxidant drugs at early hyperoxic phase of ROP may be more effective than their application to ROP at late phase.
To support this notion, we present for the first time that 17β-E2 as a nonselective ER agonist can ameliorate early hyperoxic phase of OIR (P9 and P13) , as it does on late phase (P17) of OIR [13,14,25]. Since capillary-free areas in the early hyperoxic phase are crucial for neovascularization in late phase, early 17β-E2 administration which decreases the percentage of the central capillary-free area to total retina area should be more effective in treating OIR than its late administration.
17β-E2 can bond with estrogen receptor (ER) α and β which are distributed not only in the cytosol but also on the cell membrane to exert its rapid membrane-initiated steroid signaling (MISS) , such as anti-oxidative stress, and its durable nuclear-initiated steroid signaling (NISS) , such as the transcription and expression of the VEGF gene [26,27]. It is highly possible that 17β-E2 can exert its role in ameliorating OIR in early hyperoxic phase by rapidly bonding with ERα and β on the cell membrane to exert its anti-oxidative stress function and promote retinal vessel development through MISS, and in late hypoxic phase through NISS to modulate oxidative stress and retinal neovascularization.
In sum, we found that striking alterations in retinal morphology and oxidative stress occurred in early phase of OIR. It should be more efficient to treat ROP with antioxidant drugs during the early phase than that during the late phase.
Supplementary Material
Acknowledgments
H. Zhang is supported by an internal grant from Eye Institute of Shaanxi Province and Xi’an First Hospital. X. Li is supported by an internal grant from the KUMC Kidney Institute and NIH grant R01DK084097.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Hartnett ME, Penn JS. Mechanisms and management of retinopathy of prematurity. N Engl J Med. 2012;367:2515–2526. doi: 10.1056/NEJMra1208129. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.RYAN H. Retrolental fibroplasia; a clinicopathologic study. Am J Ophthalmol. 1952;35:329–342. doi: 10.1016/0002-9394(52)90003-2. [DOI] [PubMed] [Google Scholar]
- 3.LOCKE JC. Retrolental fibroplasia definitive role of oxygen administration in its etiology. AMA Arch Ophthalmol. 1954;51:73–79. [PubMed] [Google Scholar]
- 4.Smith LE, Wesolowski E, McLellan A, et al. Oxygen-induced retinopathy in the mouse. Invest Ophthalmol Vis Sci. 1994;35:101–111. [PubMed] [Google Scholar]
- 5.Holmstrom G, Larsson E. Long-term follow-up of visual functions in prematurely born children. a prospective population-based study up to 10 years of age. J AAPOS. 2008;12:157–162. doi: 10.1016/j.jaapos.2007.08.012. [DOI] [PubMed] [Google Scholar]
- 6.Hamilton R, Bradnam MS, Dudgeon J, et al. Maturation of rod function in preterm infants with and without retinopathy of prematurity. J Pediatr. 2008;153:605–611. doi: 10.1016/j.jpeds.2008.05.018. [DOI] [PubMed] [Google Scholar]
- 7.Ecsedy M, Varsányi B, Szigeti A, et al. Cone function in children with a history of preterm birth. Doc Ophthalmol. 2011;122:141–148. doi: 10.1007/s10633-011-9268-z. [DOI] [PubMed] [Google Scholar]
- 8.Chen TC, Tsai TH, Shih YF, et al. Long-term evaluation of refractive status and optical components in eyes of children born prematurely. Invest Ophthalmol Vis Sci. 2010;51:6140–6148. doi: 10.1167/iovs.10-5234. [DOI] [PubMed] [Google Scholar]
- 9.Hartnett ME. Studies on the pathogenesis of avascular retina and neovascularization into the vitreous in peripheral severe retinopathy of prematurity (an american ophthalmological society thesis) Trans Am Ophthalmol Soc. 2010;108:96–119. [PMC free article] [PubMed] [Google Scholar]
- 10.Lange C, Ehlken C, Stahl A, et al. Kinetics of retinal vaso-obliteration and neovascularisation in the oxygen-induced retinopathy (OIR) mouse model. Graefes Arch Clin Exp Ophthalmol. 2009;247:1205–1211. doi: 10.1007/s00417-009-1116-4. [DOI] [PubMed] [Google Scholar]
- 11.Saito Y, Geisen P, Uppal A, et al. Inhibition of NAD (P) H oxidase reduces apoptosis and avascular retina in an animal model of retinopathy of prematurity. Mol Vis. 2007;13:840–853. [PMC free article] [PubMed] [Google Scholar]
- 12.Saito Y, Uppal A, Byfield G, et al. Activated NAD (P) H oxidase from supplemental oxygen induces neovascularization independent of VEGF in retinopathy of prematurity model. Invest Ophthalmol Vis Sci. 2008;49:1591–1598. doi: 10.1167/iovs.07-1356. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Miyamoto N, Mandai M, Takagi H, et al. Contrasting effect of estrogen on VEGF induction under different oxygen status and its role in murine ROP. Invest Ophthalmol Vis Sci. 2002;43:2007–2014. [PubMed] [Google Scholar]
- 14.Zhang H, Sun N, Liang H, et al. The protective effect of 17beta-estradiol on oxygen-induced retinopathy and its relation with the changes of malondiadehyde. J Biomed Res. 2010;24:138–144. doi: 10.1016/S1674-8301(10)60022-X. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Zhang HB, Sun NX, Liang HC, et al. 17-Alpha-estradiol ameliorating oxygen-induced retinopathy in a murine model. Jpn J Ophthalmol. 2012;56:407–415. doi: 10.1007/s10384-012-0136-5. [DOI] [PubMed] [Google Scholar]
- 16.D’Amato R, Wesolowski E, Smith LE. Microscopic visualization of the retina by angiography with high-molecular-weight fluorescein-labeled dextrans in the mouse. Microvasc Res. 1993;46:135–142. doi: 10.1006/mvre.1993.1042. [DOI] [PubMed] [Google Scholar]
- 17.Ohkawa H, Ohishi N, Yagi K. Assay for lipid peroxides in animal tissues by thiobarbituric acid reaction. Anal Biochem. 1979;95:351–358. doi: 10.1016/0003-2697(79)90738-3. [DOI] [PubMed] [Google Scholar]
- 18.Rinaldi Tosi ME, Bocanegra V, Manucha W, et al. The Nrf2-Keap1 cellular defense pathway and heat shock protein 70 (Hsp70) response. Role in protection against oxidative stress in early neonatal unilateral ureteral obstruction (UUO) Cell Stress Chaperones. 2011;16:57–68. doi: 10.1007/s12192-010-0221-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Souvannakitti D, Nanduri J, Yuan G, et al. NADPH oxidase-dependent regulation of T-type Ca2+ channels and ryanodine receptors mediate the augmented exocytosis of catecholamines from intermittent hypoxia-treated neonatal rat chromaffin cells. J Neurosci. 2010;30:10763–10772. doi: 10.1523/JNEUROSCI.2307-10.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Alme AM, Mulhern ML, Hejkal TW, et al. Outcome of retinopathy of prematurity patients following adoption of revised indications for treatment. BMC Ophthalmology. 2008;8:23. doi: 10.1186/1471-2415-8-23. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Kretzer FL, Mehta RS, Johnson AT, et al. Vitamin E protects against retinopathy of prematurity through action on spindle cells. Nature. 1984;309:793–795. doi: 10.1038/309793a0. [DOI] [PubMed] [Google Scholar]
- 22.Russell GA, Cooke RW. Randomised controlled trial of allopurinol prophylaxis in very preterm infants. Arch Dis Child Fetal Neonatal Ed. 1995;73:F27–F31. doi: 10.1136/fn.73.1.f27. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Gaton DD, Gold J, Axer-Siegel R, et al. Evaluation of bilirubin as possible protective factor in the prevention of retinopathy of prematurity. Br J Ophthalmol. 1991;75:532–534. doi: 10.1136/bjo.75.9.532. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Vento M, Aguar M, Escobar J, et al. Antenatal steroids and antioxidant enzyme activity in preterm infants: influence of gender and timing. Antioxid Redox Signal. 2009;11:2945–2955. doi: 10.1089/ars.2009.2671. [DOI] [PubMed] [Google Scholar]
- 25.Wang B, Zou Y, Li H, et al. Genistein inhibited retinal neovascularization and expression of vascular endothelial growth factor and hypoxia inducible factor 1alpha in a mouse model of oxygen-induced retinopathy. J Ocul Pharmacol Ther. 2005;21:107–113. doi: 10.1089/jop.2005.21.107. [DOI] [PubMed] [Google Scholar]
- 26.Miller VM, Duckles SP. Vascular actions of estrogens: functional implications. Pharmacol Rev. 2008;60:210–241. doi: 10.1124/pr.107.08002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Hammes SR, Levin ER. Extranuclear steroid receptors: nature and actions. Endocr Rev. 2007;28:726–741. doi: 10.1210/er.2007-0022. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.