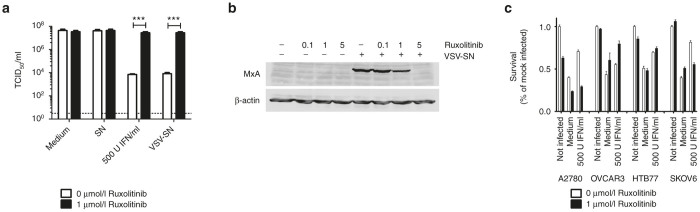
Figure 4.
Ruxolitinib can inhibit antiviral effects of IFN. (a) A2780 cells were infected with an MOI of three of the single-cycle infective, attenuated VSV*MQΔG-GP or left noninfected. Supernatants were collected 24 hours post infection. Fresh A2780 cells were treated with either medium, the supernatant of noninfected cells (SN), virus infected cells (VSV-SN), or 500 U/ml recombinant universal type I IFN. Additionally, 1 μmol/l ruxolitinib was added to half of the wells. After 18 hours, cells were infected with an MOI 0.1 of VSV-GP and 24 hours after infection, supernatants were collected and analyzed for viral replication using TCID50 assay on G62 cells. Bars represent mean ± SEM of at least two independent experiments performed in triplicates. ***P < 0.001 (Unpaired, two-tailed t-test). (b) A2780 cells were incubated with different concentrations of ruxolitinib alone (0.1, 1, and 5 µmol/l) or in combination with 1ml IFN-containing supernatant from VSV*MQΔG-GP infected A2780 cells. After 24 hours lysates were prepared and analyzed for expression of MxA. As loading control blots were probed with an antiactin antibody. (c) A2780, OVCAR3, HTB77 or SKOV6 cells were preincubated with 500 U IFN for 16 hours. Cells were subsequently infected with an MOI of 0.1 with VSV-GP or remained uninfected. Half of the wells were additionally treated with 1 µmol/l ruxolitinib. After 48 hours, viability of cells was analyzed via WST-1 assay. Bars show mean ± SEM of hexaplicates. IFN, interferon; MOI, multiplicity of infection; SEM, standard error of mean; TCID50, 50% tissue culture infective dose; VSV, vesicular stomatitis virus.