Figure 7.
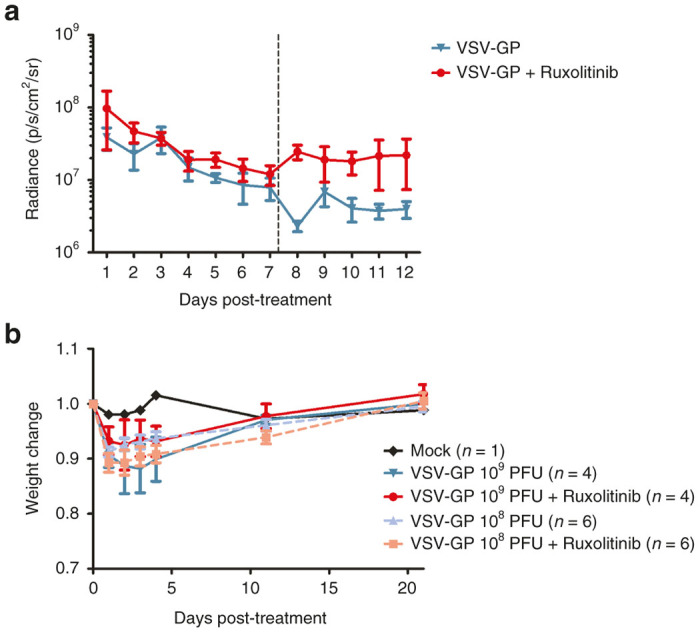
Combination of VSV-GP and ruxolitinib enhances virus replication in the tumor and is safe in immunodeficient mice. (a) A2780 tumors stably expressing luciferase were established in nude mice by subcutaneous injection. At a tumor size of 0.1 cm3 mice were either treated with VSV-GP alone or VSV-GP plus ruxolitinib. Ruxolitinib was injected once a day at a dose of 45 mg/kg ruxolitinib diluted in 200 μl methylcellulose i.p. starting 4 hours prior to the virus injection for 11 subsequent days. VSV-GP was injected at a dose of 5 × 106 PFU intratumorally on days 0 and 7. Virus replication was monitored daily via IVIS. Dashed line indicates time point of second treatment. Graph shows mean ± SEM of four (VSV-GP only) or six mice (combination group). (b) Nude mice were injected i.p. with 108 or 109 PFU of VSV-GP containing luciferase. One half of each group additionally received ruxolitinib by i.p. injection once a day (45 mg/kg) for four consecutive days starting 4 hours prior to virus injection, n = 4 for 109 PFU, n = 6 for 108 PFU, and n = 1 for mock. Mice were weighed at indicated time points. The weight of each mouse prior to virus injection was set to 1. The graph shows mean ± SEM. PFU, plaque forming unit; SEM, standard error of mean; VSV, vesicular stomatitis virus.
