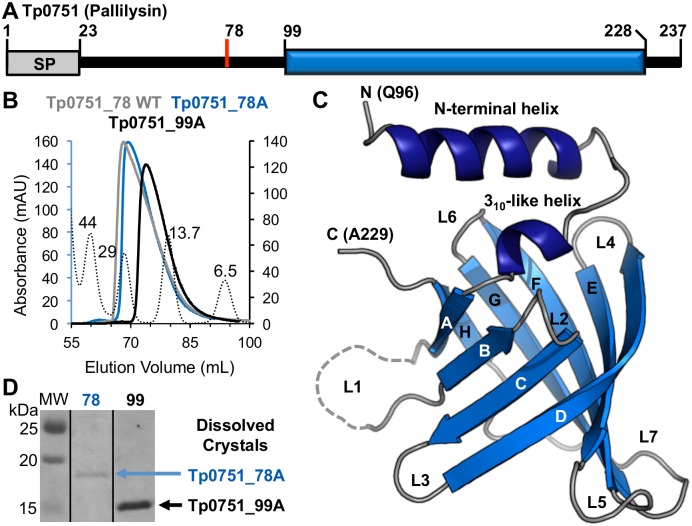
Fig 1. Tp0751 adopts a compact beta-barrel structure.
(A) Schematic of the Tp0751 primary structure, indicating signal peptide (SP, grey box), putative thrombin cleavage site (Ser78, red line), and the region with predicted secondary structure elements (Val99 to His228, blue box). (B) Size exclusion chromatograms for constructs of Tp0751 (Tp0751_78A, 18 kDa; Tp0751_99A, 16 kDa) and globular standards (dotted lines: molecular weight (MW) in kDa listed above each peak) using a Superdex 75 HiLoad column. (C) Tertiary structure of Tp0751_78A with helices in dark blue, beta-strands in marine blue, and coils in grey, revealing an eight-stranded antiparallel beta-barrel capped by two helices. Note that the most N-terminal residue modeled is Gln96. (D) To assess if the N-terminal region (residues 78 to 95) was disordered in the crystal or proteolyzed, crystals were rinsed and loaded on an SDS-PAGE gel, revealing that the complete Tp0751_78A (predicted MW of 18 kDa) construct is packed in the crystal.