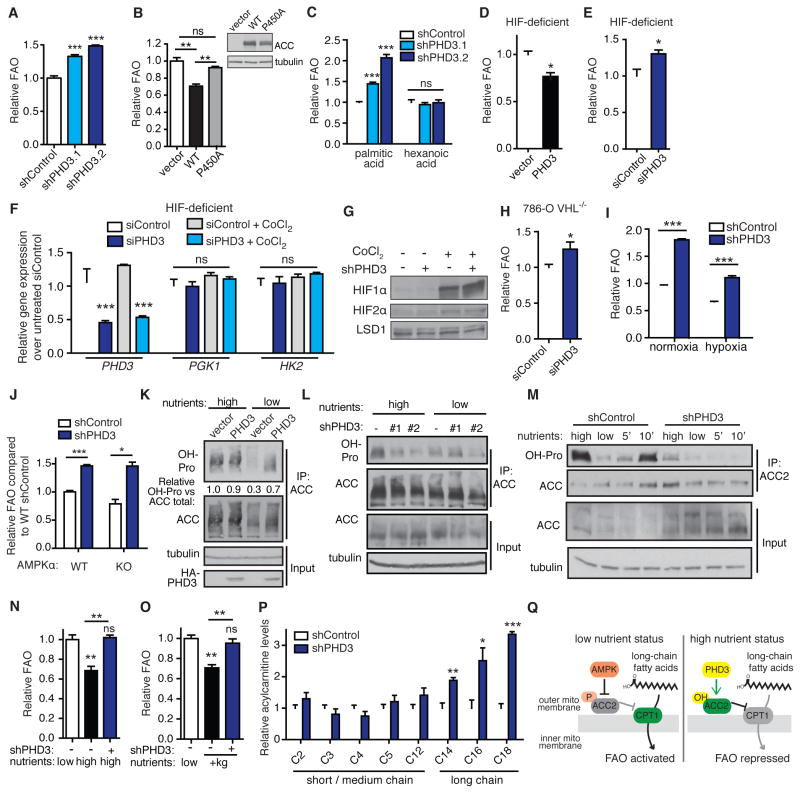
Figure 3. PHD3 represses FAO in response to nutrient abundance and independently of HIF and AMPK.
(A) Palmitate oxidation by 293T cells following stable PHD3 knockdown by shRNA (shPHD3.1 and shPHD3.2) or non-targeting control (shControl) (n = 4).
(B) Palmitate oxidation in complete medium in 293T cells overexpressing WT or mutant ACC2 (n = 3).
(C) Oxidation of long chain palmitic acid versus medium chain hexanoic acid in 293T cells expressing shPHD3 or non-targeting control shRNA (n = 3).
(D–E) Palmitate oxidation in HIF-deficient mouse hepatoma 4 (B13NBii1) cells. FAO was assessed 48 h after transfection with human HA-PHD3 or vector or with siPHD3 or siControl (n = 3).
(F) Validation of HIF deficiency in HIFβ-null hepatoma cells. PHD3 expression and HIF target gene expression in HIFβ-deficient cells transfected with siRNA against PHD3 or control, and treated with or without the HIFα-stabilizing compound CoCl2 (250 μM, 6 h) (n = 4).
(G) Immunoblot of HIF1α and 2α levels in 293T cells with PHD3 knockdown or control. HIFα was made more identifiable by 6 h treatment with 250 μM CoCl2 in separate control samples.
(H) Effect of PHD3 knockdown on palmitate oxidation in 786-O VHL−/− cells (n = 3).
(I) Palmitate oxidation in 293T cells following 12 h pre-incubation in normoxia or hypoxia (1% O2). Cells were maintained under normoxia or hypoxia during FAO analysis (n = 4).
(J) Palmitate oxidation in WT versus AMPKα KO MEFs expressing shRNA against PHD3 or non-silencing control (n = 3).
(K) ACC hydroxylation in 293T cells following 12 h incubation in high versus low nutrient medium. High nutrient DMEM contains 4.5 g/L glucose and serum. Low nutrient DMEM contains 1 g/L glucose without serum. ACC was immunoprecipitated and hydroxylation was detected by immunoblot.
(L) 293T cells expressing shRNA against PHD3 or control were incubated 12 h in high or low nutrient media prior to analyzing ACC hydroxylation.
(M) ACC hydroxylation dynamically responds to cellular nutrient cues. WT immortalized MEFs were incubated in high or low nutrient medium for 6 h, or in low nutrient medium for 6 h followed by adding back high nutrient medium for 5 or 10 min. ACC-IP was performed in lysis buffer containing the PHD inhibitor DMOG (1 mM) to minimize hydroxylation in the lysis buffer. Hydroxylation was detected by immunoblot.
(N) Impact of PHD3 knockdown on the ability of MEFs to modulate FAO in response to low or high nutrient medium (n = 3).
(O) Impact of PHD3 knockdown on the ability of 293T cells to suppress FAO in response to supplementing low glucose, serum-free medium with dimethyl ketoglutarate (+kg, 5 mM) for 6 h prior to and during 2 hr FAO analysis (n = 3).
(P) Acylcarnitine levels measured by metabolomics analysis of 293T cells grown in high nutrient medium following stable knockdown of PHD3 or control. Levels were normalized to cell count in parallel plates (n = 6 for control, n = 3 for shPHD3).
(Q) Two-part model of ACC2 regulation. Under low nutrient conditions, AMPK responds to the AMP/ATP ratio to phosphorylate and inhibit ACC2, thus promoting long chain FAO. Under high nutrient conditions, PHD3 hydroxylates and activates ACC2 to limit long chain FAO.
*p < 0.05, **p < 0.01, ***p < 0.001. Data represent mean ± SEM. See also Figure S2.