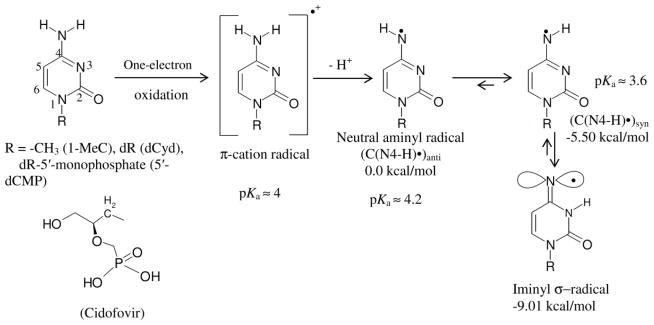
Scheme 5.
This scheme represents C•+ production via one-electron oxidation of cytosine derivatives and the prototropic equilibria of C•+ in these compounds. By deprotonation and subsequent tautomerization, the C•+ π-radical forms the iminyl σ-radical. The atom numbering scheme of cytosine base is presented. The relative stabilities of C(N4-H)•syn, C(N4-H)•anti and the iminyl σ-radical in kcal/mol as well as the pKa values (Close, 2013) of C•+, C(N4-H)•syn, C(N4-H)•anti are provided. The energy values were obtained with the DFT/B3LYP/6-31G* method using geometry optimized structures. Reprinted with permission from Adhikary et al. 2015, J. Phys. Chem. B, copyright (2015), American Chemical Society.