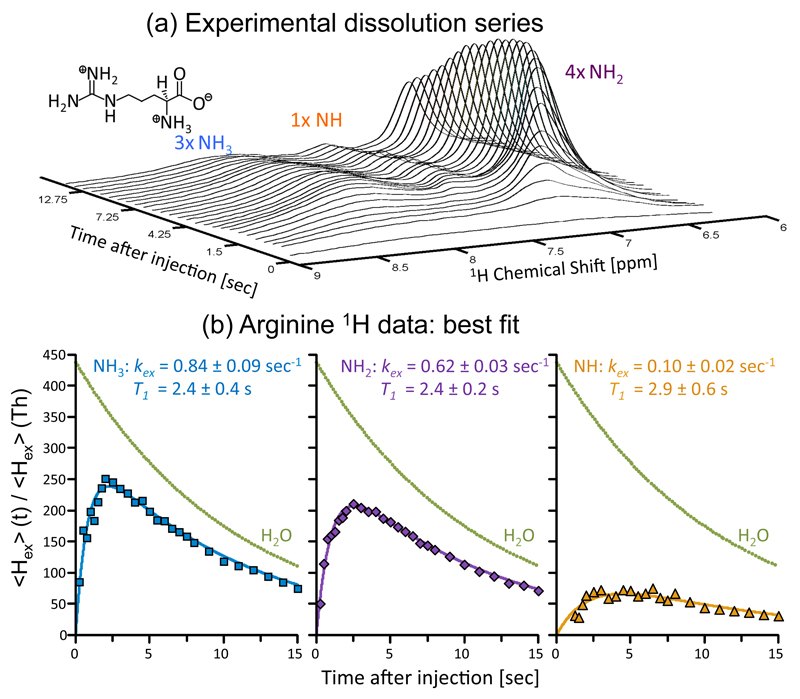
Figure 4.
Transferring water hyperpolarization to the resonance of arginine’s exchangeable protons. (a) Progression of arginine’s 1H NMR spectrum upon sudden dissolution of hyperpolarized water into 3 mL of a 1M arginine sample (pD ~ 3) dissolved in D2O, and waiting in the 500 MHz spectrometer used to collect the data. Each trace involved the acquisition of 4k complex points, arising from a small flip-angle (~1°) excitation (carrier at 7.3 ppm) with a 0.25 sec TR. The different types of protons in the arginine sample (inset - molecular formula) are indicated above their corresponding peaks. (b) Peak intensities arising from the experimental time course, together with fits to Eq. (2) for each arginine site (solid lines), lead to the indicated relaxation times T1 and exchange rates kex. These fits revealed an initial water polarization enhancement of (438±3)×, and a characteristic decay T1(H2O) of 10.9 ± 0.1 sec; the ensuing decay curve is presented in the figure as green dots).