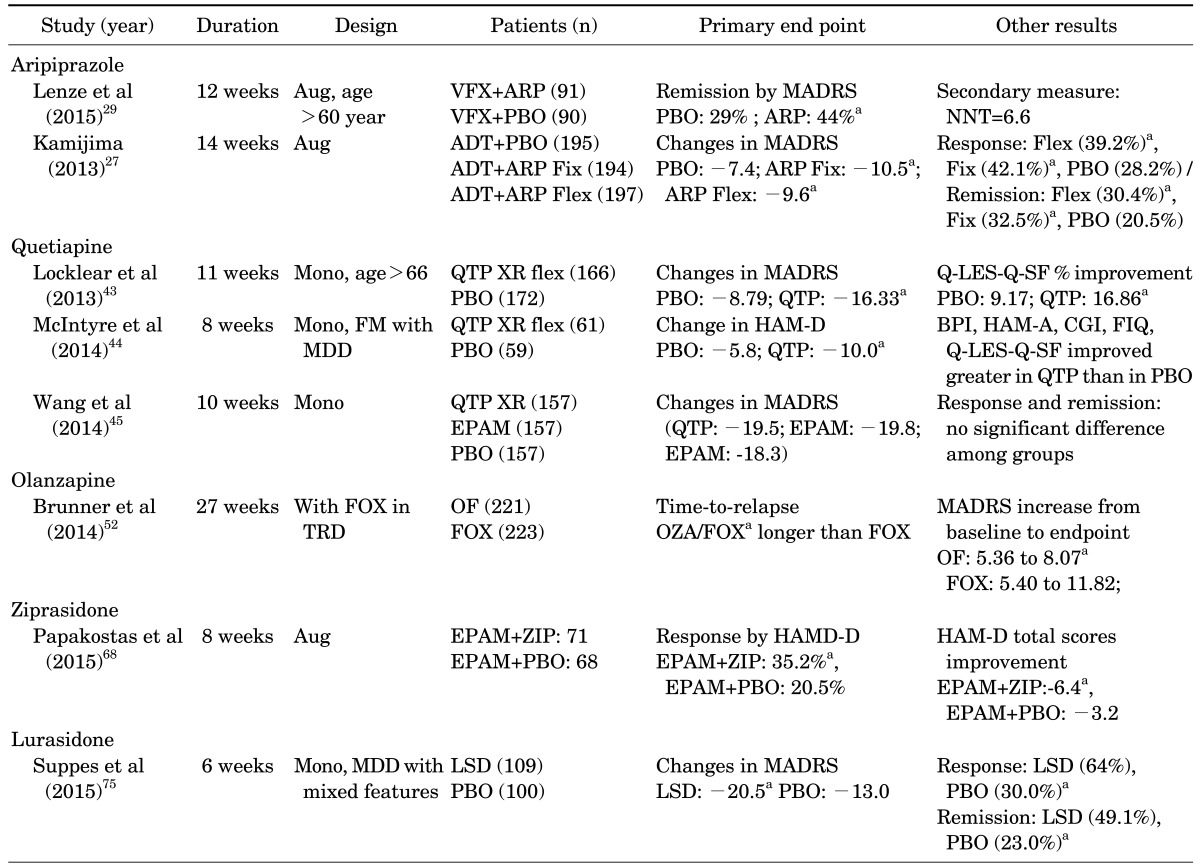
TABLE 3. Randomized, double-blind, placebo-controlled trials of SGAs in major depressive disorder since 2013.
ap<0.05 vs placebo, otherwise not significant.
ARP: aripiprazole, Aug: augmentation, BPI: brief pain inventory short form, EPAM: escitalopram, FIQ: fibromyalgia impact questionnaire, Fix: fixed dosing, Flex: flexible dosing, FM: fibromyalgia, FOX: fluoxetine, GAS: global assessment scale, HAMD-D: 17-item hamilton depression rating scale, LSD: lurasidone, MADRS: montgomery-asberg depression rating scale, Mono: monotherapy, NNT: number needed to treat, OF: olanzapine and fluoxetine combination, PBO: placebo, PHQ: patient health questionnaire, Q-LES-Q-SF: quality of life enjoyment and satisfaction questionnaire short form, QTP: quetiapine, XR: extended release, ZIP: ziprasidone.