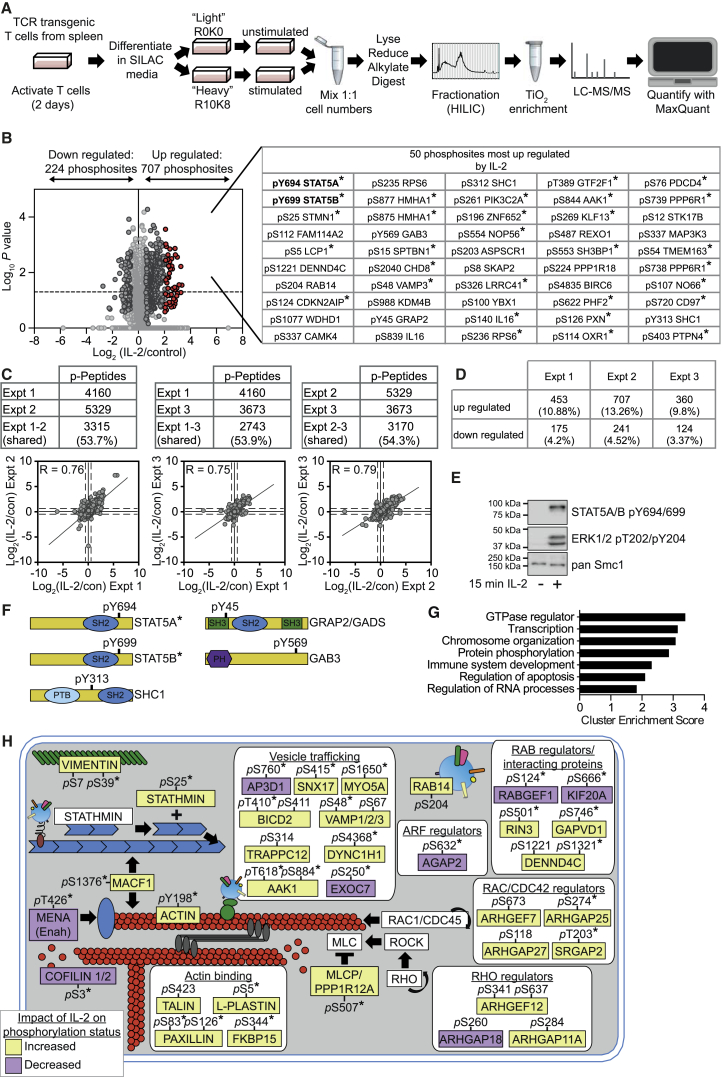
Figure 1.
Phosphoproteomic Analysis of IL-2 Maintained CTL
(A) Experimental workflow for SILAC-based quantitative phosphoproteomic analysis of T cells.
(B) CTLs differentiated as in (A) were starved of IL-2 for 24 hr in the presence of IL-12 to sustain expression of CD25 (IL-2 quiesced). The heavy-labeled CTLs were stimulated with 20 ng/mL IL-2 for 15 min mixed with the control (light) cells, and phosphopeptides were prepared. Phosphosites identified in three biological replicates are shown, with log-transformed SILAC ratios plotted against log-transformed p values (one sample t test); refer to Tables S1 and S2 for identified phosphosites. Phosphosites with ratios reproducibly changed by 1.5-fold are shown in dark gray. The 50 phosphosites most reproducibly increased by IL-2 are shown in red and are displayed alongside. Phosphosites found to show a statistically significant regulation (p value ≤ 0.05) are marked with an asterisk (∗).
(C) The overlap and correlation in the SILAC ratios of the phosphosites identified in the individual biological replicates is shown.
(D) The numbers and percentages of phosphosites regulated in each replicate are shown.
(E) CTL deprived of IL-2 and maintained in IL-12 were treated with or without IL-2 for 15 min and analyzed for STAT5 and ERK phosphorylation by immunoblot.
(F) A schematic representation of the IL-2-regulated phospho-Tyr residues is shown.
(G) The proteins regulated by phosphorylation in response to IL-2 were evaluated for function. The graph shows the cluster enrichment score as determined by DAVID analysis. See also Table S3.
(H) Overview of selected phosphosites regulated consistently by IL-2 in two or more experiments in proteins that regulate the cytoskeleton or vesicle transport.