Table 4.
Gold nanoparticles (AuNPs) responsive to laser via surface plasmon resonance (SPR).
| AuNP Structure and Size | In Vitro Effects | In Vivo Effects | Ref. |
|---|---|---|---|
 25–150 nm |
SPR peaked between 500 and 1000 nm. The dependence of heat efficiency on size and wavelength decreased after cellular uptake (PC3 cells). | The dependence of heat efficiency on size and wavelength decreased only after cellular uptake (intratumoral injection of Au stars, PC3 xenograft). | [69] |
 Branched AuNPs Deoxycholate concentration-dependent particle size |
cRGD-branched AuNPs decreased cell viability on BxPC3 cells after photothermal ablation (NIR laser source at 808 nm, 1.4 W/cm2, for 3 min). | cRGD-branched AuNPs + NIR laser irradiation had the best antitumor effect on BxPC3 xenograft. | [70] |
 Raw carbon nanotubes 1–2 nm Size of final product varied. |
Increased cytotoxicity in combination with NIR irradiation at 808 nm for 15 min (1.6 W, spot size 5 × 20 mm2, HeLa cells). | None | [71] |
 Nanorods 60 × 14.8 ± 6.5 × 2.0 nm  Nanocage edge length 50 ± 7 nm |
Compared to nanorod, nanocage had higher light-to-heat transduction efficiencies and higher cellular uptake (HUVEC and DU145 cells). | Compared to nanorod, nanocage had more optimal biodistribution profile over time and higher excretion rate. | [72] |
 |
TAT facilitated cellular uptake. Higher photothermolysis efficiency on BT549 breast cancer cells (850 nm pulsed laser source under 0.2 W/cm2 irradiation). | None | [73] |
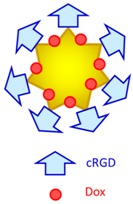 Au nanostar 32.6 nm Au-cRCD-Dox 124.7 nm |
cRGD facilitated cellular uptake. Synergistic effect of photothermal therapy and chemotherapy (765 nm high power multimode pump laser, 1.0 W/cm2, 10–15 min, MDA-MB-231 and Bel-7402 cells). | Prominent accumulation in tumor and reticuloendothelial system in the liver, and synergistic effect of photothermal therapy and chemotherapy (S180 xenograft). | [74] |
 47 nm |
Enhanced photothermal therapy outcome on Mucin-7-expressing MBT2, T24, 9202, and 8301 cells at low energy levels (500 exposures, 532 nm laser) | None | [75] |
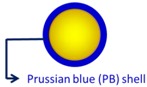 AuNP 9.1 ± 0.64 nm Au@PB NPs 17.8 ± 2.3 nm |
Enhanced photothermal cytotoxicity (HeLa cells, NIR 808 nm laser, 1.5W/cm2, 10 min). Concentration dependent X-ray, CT, and photoacoustic signals. |
Enabled photothermal ablation and simultaneous photoacoustic/CT bimodal imaging (HT-29 xenograft). | [77] |
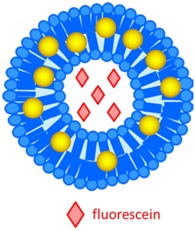 AuNP 3–7 nm AuNP-liposome 100 nm |
Laser induced disintegration of liposome and triggered release of fluorescein (fiber-optic guided 65 mW laser, 532 nm). | Higher tumoral retention of fluorescein by liposome as compared to free fluorescein, and fluorescein release triggered by laser (MDA-MB 231 cell xenograft). | [78] |
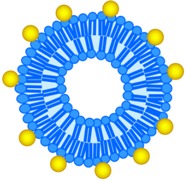 LiposAuNPs 100–120 nm |
LiposAuNPs were biocompatible on NIT-3T3 cell line, but exhibited cytotoxicity in combination with laser irradiation (MCF-7 and HT1080 cells). | In situ degradation in hepatocytes and clearance through hepatobiliary and renal routes. Complete tumor ablation using NIR laser (750 nm). | [79] |
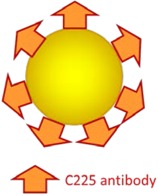 60 nm |
Intracellular synergy by (1) nanocluster formation after cellular internalization of AuNPs and TNs; (2) release of the chemo agent upon receiving laser pulse by generation of plasmonic nanobubbles; (3) amplification of X-ray. (HN31 cell lines). | Quadrapeutics system including AuNPs, TNs, laser, and X-ray had the most improved efficacy on fast-growing aggressive HN31 xenograft, as compared with standard chemoradiation. | [80] |
 35 nm |
Hydrogel shell formation on cells. Enhanced cytotoxicity via combination of photothermal therapy and photodynamic therapy (808 nm, 200 mW/cm2, HeLa and Chinese hamster ovary cells). | None | [81] |
 AuNP 5 nm, FA- miR-122-AuNP 20 nm, GO and GGMPN nanocomposites 500 nm |
P-gp antibody and FA facilitated cell targeting. Increased apoptosis on drug-resistant HepG2 cells. | Apoptosis induction and tumor growth inhibition on HepG2 xenograft (semiconductor laser light source, 10 min, every 2 days, 10 treatments). | [82] |
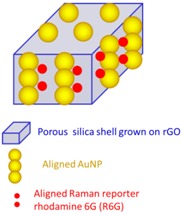 rGO (< 200 nm) Thickness of GO/silica nanosheets 44 nm, AuNP 4 nm |
Anti-EGFR SERS probe nanocomposite. Cancer cell tracking by Raman imaging. Enhanced cytotoxicity by synergistic photothermal effect of AuNP and rGO (808 nm laser, 0.5 W/cm2, A549 cells). | None | [83] |
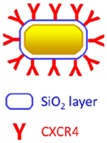 Nanorods length 65.0± 7.5 nm and width 12.0 ± 1.5 nm |
Loading of nanorods@ SiO2@CXCR4 into human iPS cells. Reservation of viability of iPS cells and photothermal property of Au nanorods. |
Stem cell mediated tumoral delivery, MGC803 xenograft. Prolonged tumoral retention confirmed by photoacoustic and two-photon luminescence imaging. Tumor growth inhibition via photothermal therapy (NIR laser at 808 nm 1.5 W/cm2) |
[84] |
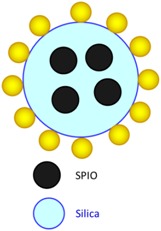 SPIO 10 nm SPIO@AuNPs 82 ± 4 nm |
Loading of SPIO@AuNPs into AD-MSCs. Reservation of viability of AD-MSCs and photothermal and magnetic properties of SPIO@AuNPs. Photothermal ablation of HepG2 cells by SPIO@AuNP–loaded AD-MSCs. |
Homing of AD-MSCs to liver injuries or HCC confirmed by MR imaging and histologic analysis. | [85] |
 30–50 nm |
None | Proved NPs interact with ablative techniques differently. Cellular incorporation of NP was only observed after combination with irreversible electroporation. Structural deformation was only observed in combination with laser-induced thermal therapy (808 nm NIR laser). | [68] |
cRGO, cyclic RGD; NIR, near infrared; TAT, TAT peptide; Dox, doxorubicin; PB, Prussian blue; CT, computed tomography; TN, therapeutic nanoparticle; P-gp, P-glycoprotein; FA, folic acid; miRNA, GO, graphene oxide; SERS, surface-enhanced Raman spectroscopy; EGFR, epidermal growth factor receptor; rGO, reduced graphene oxide; iPS, human induced pluripotent stem cells; MR, magnetic resonance; HCC, hepatocellular carcinoma; Ad-MSCs, adipose-derived mesenchymal cells; GGMPN, gold nanoparticles loaded with miR-122; SPIO@AuNPs, superparamagnetic iron oxide-coated gold nanoparticles.
