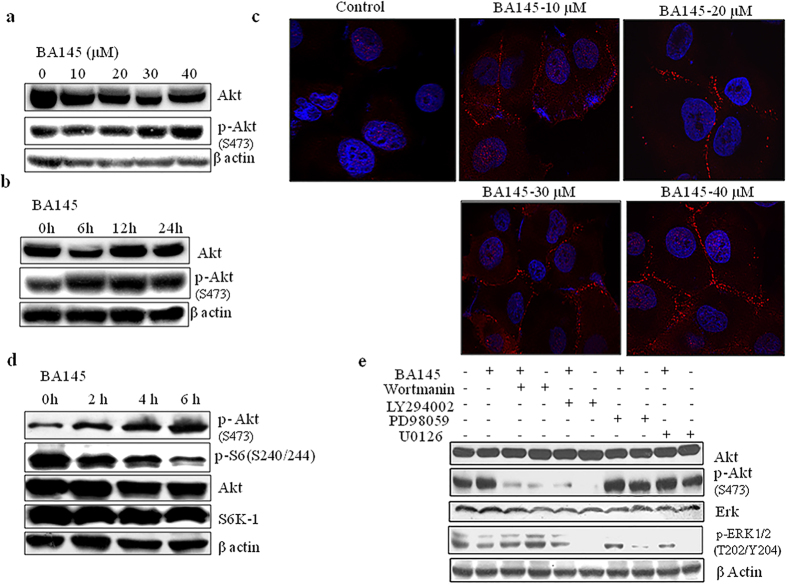
Figure 6. Inhibition of mTOR signaling by BA145 leads to an increase in Akt phoshorylation in PANC-1 cells.
(a) PANC-1 cells were treated with BA145 at indicated doses for 24 h and then lysed in lysis buffer for western blot analysis phosphorylation and expression of Akt (b) Time dependent increase in p-Akt expression (serine 473) in BA145 (40 μM) treated PANC-1 cells (c) BA145 induced membrane translocation of Akt (red arrow heads) in PANC-1 cells in dose dependent manner. Cells were treated with BA145 at indicated doses for 24 h and then immunofluorescence staining was performed using fluorescent p-Akt (serine 473) antibody (d) Western blot analysis of the Akt, S6 kinase and their phosphorylated forms in BA145 (40 μM) treated PANC-1 cells at indicated time intervals (e) Involvement of PI3kinases in BA145 mediated activation of Akt. PANC-1 cells were pre-treated with PI3Kinase inhibitors wortmanin (1 μM) and LY294002 (30 μM) and MEK/Erk pathway inhibitors PD98059 (40 μM) and U0126 (20 μM) for 1 h and then cotreated with BA145 (40 μM) for another 5 h. The cells were subjected to the preparation of whole cell protein lystaes for detection of the indicated proteins using western blotting. PI3kinase inhibitors blocks the phosphorylation of Akt at serine 473 induced by BA145 whereas, Mek/Erk pathway inhibitors have no any effect of Akt activation.