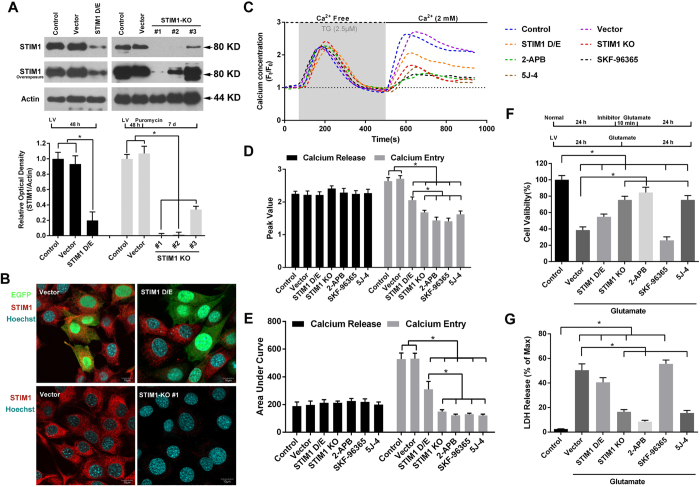
Figure 4. SOCE inhibition by knocking out of STIM1 or application of SOCE inhibitors attenuated glutamate-induced HT-22 cell injury.
HT-22 cells were infected with lentivirus particles containing either scramble-shRNA (Scr-shRNA), or STIM1-shRNA for 48 h and harvested for western blotting assay (A, Left) to validate the efficiency of STIM1 down-regulation. For knockout of STIM1 expression, HT-22 cells were infected with CRISPR/Cas9-mediated particles with either vector or a STIM1 KO construct (#1, #2, and #3) for 48 h and then conditionally cultured with puromysin for 7 days. The expression of STIM1 was detected and represented as percent optical density of control cells (A, Right). STIM1 protein levels for cells treated with Scr-shRNA, STIM1-shRNA (MOI = 5), vector, or STIM1 KO #1 were also examined using immunofluorescence staining (B). After verification of the efficiency of STIM1 down-regulation or knockout, the HT-22 cells were loaded with Fura-red (4 μM) and baseline levels were recorded every 15 s for a total of 100 s. The HT-22 cells were then treated with 2.5 μM TG to release Ca2+ from calcium stores (gray zone) in Ca2+-free buffer (black dotted line) for 400 s. The buffer was then refreshed with Ca2+buffering (2 mM, black full line). Changes in Ca2+ signals were evaluated as time-series curve (C) and assessed as peak F/F0 (D) and area under curve (AUC, E). Approximately 40 to 50 cells were analyzed in each experiment. Twenty-four h after normal or lentivirus-infected HT-22 cells were seeded into 96-well or 6-well plates with identical cell density, the cells were pretreated with 2-APB (100 μM), SKF96365 (25 μM), or 5J-4 (20 μM) for 10 min, followed by treatment with glutamate. Cell viability (F) and LDH release (G) were assessed 24 h later. Data are presented as mean ± SEM from four experiments. *p < 0.05.