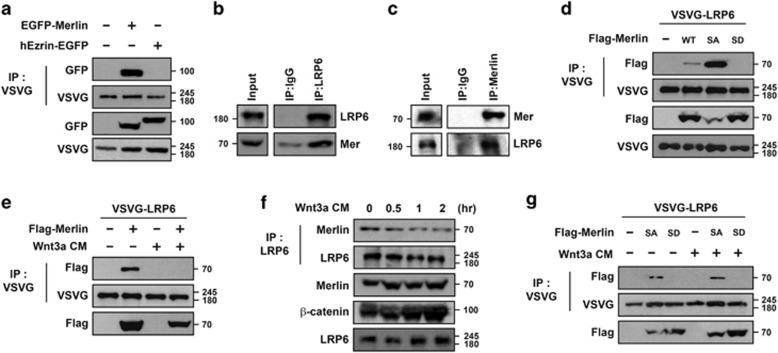
Figure 1.
Interaction of Merlin with LRP6 is disrupted upon Wnt signaling. (a) Merlin bound to LRP6 under overexpression conditions. VSVG-LRP6 together with EGFP-Merlin or hEzrin-EGFP was transfected into HEK293T cells, subjected to immunoprecipitation with anti-VSVG, and immunoblotted with the indicated antibodies. (b and c) Merlin bound to LRP6 at endogenous level. MDCK cells were lysed, subjected to immunoprecipitation with anti-LRP6 (b), anti-Merlin (c), or control mouse IgG antibodies, and immunoblotted with the indicated antibodies. (d) Phospho-mimetic form of Merlin did not interact with LRP6. VSVG-LRP6 was co-transfected with wild-type (WT), S518A (SA), or S518D (SD) Merlin as indicated, subjected to immunoprecipitation with anti-VSVG, and immunoblotted with the indicated antibodies. (e) Interaction between Merlin and LRP6 was abrogated in the presence of Wnt. VSVG-LRP6 with or without Flag-Merlin-expressing HEK293T cells was treated with Wnt3a-CM overnight, followed by immunoprecipitation with anti-VSVG antibody and western blotting. (f) Interaction between endogenous Merlin and LRP6 was reduced upon treatment with Wnt3a-CM in a time-dependent manner. HEK293T cells were treated with Wnt3a-CM for the indicated time, followed by immunoprecipitation with anti-LRP6 antibody and western blotting with the indicated antibodies. (g) Interaction between unphospho-mimetic form of Merlin and LRP6 was not abrogated upon treatment with Wnt3a-CM. HEK293T cells, transfected with indicated plasmids, were incubated with or without Wnt3a-CM, followed by immunoprecipitation with anti-VSVG antibody and western blotting with the indicated antibodies. All western blotting and immunoprecipitation experiments were performed more than thrice and these data are representative of them