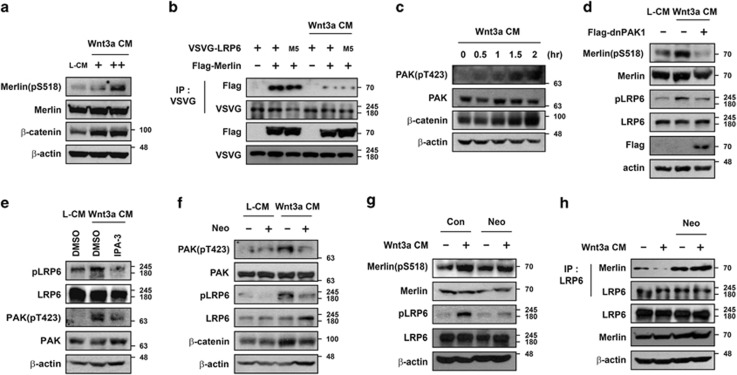
Figure 5.
Phosphorylation of Merlin upon Wnt3a treatment is mediated by PAK in a PIP2-dependent manner. (a) Treatment with Wnt3a-CM induced phosphorylation of Merlin. HEK293T cells were treated with L-CM or Wnt3a-CM (+, 2-fold diluted CM; ++, original CM) overnight and harvested, followed by western blotting with indicated antibodies. (b) Reduced interaction between LRP6 and Merlin upon Wnt3a-CM treatment is irrelevant to the phosphorylation status of LRP6. HEK293T cells transfected with VSVG-LRP6, VSVG-LRP6-M5, or Flag-Merlin were treated with Wnt3a-CM overnight and subjected to immunoprecipitation with anti-VSVG antibody. (c) Phosphorylation of PAK increased upon Wnt3a-CM treatment. HEK293T cells were treated with Wnt3a-CM at the indicated time and harvested, followed by western blotting with indicated antibodies. (d) Ectopic expression of the dominant-negative form of PAK1 (dnPAK1) inhibited Wnt3a-mediated phosphorylation of LRP6 and Merlin. After empty vector or dnPAK1 was transfected, cells were treated with L-CM or Wnt3a-CM for 2 h before being harvested, followed by western blotting with indicated antibodies. (e) Inhibition of PAK activity blocked Wnt3a-mediated phosphorylation of LRP6. Cells were pretreated with DMSO or IPA-3 (30 μM) and incubated with L-CM or Wnt3a-CM for an additional 2 h before being harvested. (f and g) Blocking of PIP2 formation inhibited Wnt3a-mediated phosphorylation of PAK (f), Merlin (g), and LRP6. HEK293T cells were treated with 10 mM neomycin for 30 min before incubation with Wnt3a-CM and neomycin for an additional 2 h. (h) Blocking of PIP2 formation inhibited Wnt3a-mediated dissociation of Merlin from LRP6. HEK293T cells were treated with 10 mM neomycin for 30 min before incubation with Wnt3a-CM and neomycin for an additional 2 h, subjected to immunoprecipitation with anti-LRP6 antibody, and immunoblotted with the indicated antibodies. All western blotting and immunoprecipitation experiments were performed more than three times, and these data are representative