Fig. 1. Model of gp96 cancer-associated clientele.
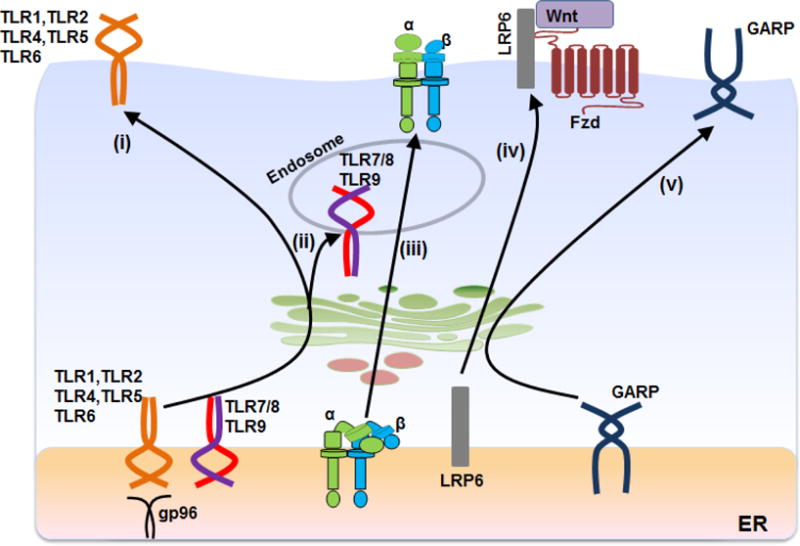
Gp96, a resident ER protein chaperones TLR1, TLR2, TLR4, TLR5 and TLR6 through the Golgi apparatus to the cell surface (i) and TLR7, TLR8 and TLR9 to endosomes (ii). Gp96 also chaperones multiple integrins (αβ subunits) (iii) and participates in canonical Wnt signaling by folding the fizzled co-receptor, LRP6 (iv). Recently, gp96 was also shown to be the key molecular chaperone for GARP (v). For clarity only relevant molecules are depicted.
